Objectives of this study
The hornet is known to forage at a distance of several kilometers from
the nest. For this purpose well developed orientation and navigation systems
are needed. In the past some observations and behavioral experiments concerning
the problems of orientation and navigation were performed. From these it was
deducted that the orientation capacities towards gravity and light are temperature
dependent. Therefore, we started our research by investigating the thermoregulation
and the source of electric energy of the hornet, whereafter the specific organs
of graviception were studied in more detail, in order to formulate our concept
of orientation and navigation of the hornet and the role the ocelli. In chapter
1 an overview is given of the various concepts, macroscopic and ultramicroscopic
structures dealt with in this study. In chapter 2 and part of chapter
3, the thermal homeostasis is discussed; thermoregulation is achieved by
the well known thermoelectric mechanism (Seebeck). In chapter 3, the
source of electric energy is dealt with, which is a photovoltaic system. In
chapter 4, we describe the micromorphology of the sensory cells of the
Ishay organ, the main organ of graviception. In chapter 5, the micromorphology
of the ocelli and its function as a main organ in dim light is discussed. In
chapter 6
we discuss the issues of orientation and navigation of the ocelli and the
surrounding organs.
Appearance of Vespa orientalis
The Oriental hornet Vespa orientalis (von Linné, 1771) is a social
insect which founds an annual (or semiseasonal) colony during the spring. These
colonies usually prevail during the warm season only and are all comprised most
of the time, of females only, as well as the progeny of one fertile female,
namely, the queen. Only during autumn, just before termination of the life cycle,
do males make their appearance. The complete life cycle involves thefollowing
stages, to wit: a fertilized queen which emerges from hibernation during February-March
to found a nest in an empty cavity, usually an abandoned rodent burrow, where
it proceeds to oviposet her first eggs in an embryo comb and nurses these till
eclosion of the initial workers. Thereafter, the handling of the brood is gradually
relegated to the workers, while the queen engages henceforth only or primarily
in oviposition.
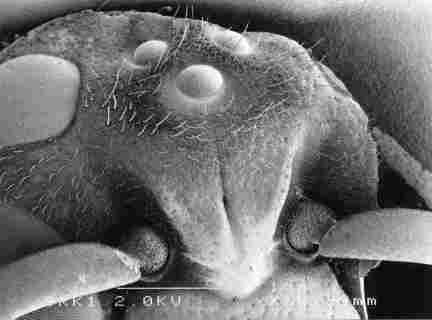
Fig. 1, 2: Overview of the head of the hornet Vespa orientalis, observed from
the outside, with two compact eyes (L, R), ral ocelli (a, b) and one medial
ocellus, vertex (vt), snout with frons plate (fp) and two antennae (an). Bar=
verview of inside of the head after removing part of its contents showing lateral
(a) and central ocellus (c), lateral ocellus hidden by hair cells. Particulary
at the periphery muscle fibres (mf). The frons plate (fp) is
with hair cells, which are barely visible at this magnification. Bar= 1 Ám.
Much information has accumulated in recent years on the biology of this and
related species or genera of hornets and wasps (Ishay, 1967; Wilson, 1971; Guiglia,
1972; Spradbery, 1973; Edwards, 1980; Matsuura and Yamane, 1990). It turns out
that the life cycles of the various species are fairly similar, except for some
primarily local or ecological differences. The salient, common features among
these hornets are the following : (a) all of them are predators, and the larger
species within the genus Vespa constitute in their area of distribution
an enemy of the honeybee. (b) all of them are estival, so that by winter only
fertilized queens survive, which are intended to found a new colony in the spring.
(c) if the hornets are active outside the nest mainly during the daytime and
in the evenings in the summer; an activity started during the daytime may continue
to completion in the dark. Darkness prevails within the nest, as well as a rather
fixed temperature and humidity, and these conditions are strictly controlled
or adhered to by the resident hornets. Furthermore, all the combs constructed
within the nest are orientated in the direction of the earth's gravitational
force.
The importance of illumination to vespan life
It was found (Ishay et al., 1980) that optimal hornet longevity
occurred under outside daylight illumination; second-best was under room daylight
illumination. Under these conditions hornets, Vespa orientalis, lived
significantly longer than under constant light or darkness. Addition of proteins
to the diet usually did not contribute to the longevity beyond that obtained
with a sugar diet alone, but did promote enhanced building of cells and better
nursing of the brood. Vespa orientalis hornets perceive the conditions
prevailing around them via sensory organs attached to their cuticle. Their various
external sense organs are covered with a layer of cuticle so that the latter
mediates between them and the environment. The cuticle thus is an active mediator
and it is therefore worthwhile to attempt to measure or assess the disparate
properties of such cuticle. In an earlier report we described the photoconductive
phenomenon which occurs on the "yellow strips" of the cuticle of Vespa
orientalis (Ishay and Croitoru, 1978). It was shown that following an initial
exposure to light of the yellow strips, of living as well as dead hornets, their
resistance increases until it reaches a saturation value. At this stage the
irradiated cuticle of the hornet acts like an organic photoconductor (Inokuchi
and Akamatu, 1961), i. e. the cuticular resistance increases when the light
is turned off and then decreases upon renewed illumination; this effect proved
to be reversible.
We were interested in investigating whether the observed photoconductive phenomenon
conforms to the general behavior of organic semiconductors under the same conditions.
What is the possible impetous of the photoconductivity in the yellow strips
on the daily activity of the hornets ? In a 'Vespa orientalis' nest,
a fairly high and fixed temperature (29-30 ░C) is usually present (Ishay et
al., 1967; Ishay and Ruttner, 1971; Ishay, 1973). The nest is situated in
darkness or at least no direct penetration of light occurs. Thus, the hornet
has an optimal temperature within its nest for carrying on its diurnal activities,
and when exposed to sunlight, outside the nest it has the readiness for conductivity
of an intrinsic semiconductor (Ishay et al., 1980). It follows, then, that this
photoconductive property of the cuticle is dependent also on the temperature,
which prompted us to assess the changes in the vespan cuticle resistance to
electric current in relation to temperature. In these investigations of the
electrical resistance of hornet cuticle a correlation was found between temperature
and cuticular resistivity in a relatively large group of hornets (49 in all),
which again points to the cuticle as being an organic semiconductor (Meier,
1974; Sachse, 1975). This basic property of the hornet cuticle is dependent
neither on the pigment color nor on the age of the hornet.
Cuticular resistance
Measurements in the course of this study were all made in the dark.
There is a difference in the electrical resistance of vespan cuticle exposed
to the same temperature, depending when the measurements are made during warming
or during cooling. This phenomenon hysteresis appears only when measurements
are made in the dark. Investigations on this phenomenon in hornets have revealed
the existence of a hysteresis loop, which is obtainable upon measurement of
a complete cycle within any temperature range between -35 ░C and + 50 ░C (Ishay
et al., 1982; Ishay and Shimony, 1983; Shimony and Ishay, 1984). This has been
defined as "thermal hysteresis ". Some insects have proteins which produce a
thermal hysteresis (Ishay et al., 1985). The worker hornets do not hibernate
and it seems that the occurrence of thermal hysteresis in the hornet's cuticle
points to the possibility that their cuticle is endowed with memory, since at
the very same temperature the resistance can differ depending on whether the
cuticle is in the process of cooling or warming. It is likely that the sentinel
hornets (Ishay et al., 1967), when probing the entering or departing hornets
with their antennae, can gauge, among other things, the resistance level of
their cuticles and thereby possibly the protocol of their flights as well as
their feeding status. Thus, it appears that the normal cuticle, beside reacting
to electrical current, as do organic semiconductors, has an advantage over the
latter in that it stores some kind of memory and therefore provides information
to nestmates. The origin of this behavior of the cuticle resides probably in
the polarity of some magnetic (or electric) elements in it (some magnetic or
electric domains). The "fat" hysteresis loop obtained between the warming and
the cooling lines in the control experiment (i. e. the space between these two
lines) represents the energy investment (or gain) in these cuticular elements
which, by changing their polarity and their spatial orientation or both, set
up a net magnetization parallel to the field applied to them (Rosenzweig et
al., 1985).
Resistance is an important feature of semiconductors, however, to understand
the biological importance of hornet cuticle as a semiconductor, there was need
to perform also measurements of the spontaneous voltage and current, that is,
the voltage and current not created by the cuticle in response to an external
electric current, inasmuch as these measurements can inform us regarding the
intrinsic properties of the cuticle as a semiconductor. Additionally, we examined
also the response of vespan cuticle to charging by an external voltage source.
Thermoelectric and photoelectric currents
The spontaneous current in the cuticle of the studied specimens ranged
between 30-40 nAmp under conditions of darkness, whereas under illumination
the current drops to near zero. Upon warming up to 28-29 ░C, the current rises
to 50-200 nAmp, however, after a while, it declines, regardless of whether the
temperature is held steady, continues to rise or is lowered. In light, the current
values are lower than in darkness and this under all conditions. When the specimen
is charged with an electric current under fixed temperature, the current attains
several nAmp in darkness, but is usually less than that under illumination by
about one order of magnitude. The capacitance values range between 1-7 mFarad
both in light and in dark. Within the temperature range used by us (8 30 ░C),
we found the cuticular response to be negative photoconductivity (Ishay et
al. 1987), meaning that upon exposure to light, the electrical conductivity
decreases (while the resistance increases). The same could be observed when
charging the cuticle with electrical current, namely, that charging in the dark
attains discharge current values which are by one order of magnitude higher
than those during charging in light. Interestingly, the capacitance values under
both conditions are not very different and generally are in the same range as
those published later for the hornet cocoon silk caps (Ishay et al., 1994).
The rise in electric current upon increase in temperature is intrinsic, in
that with increasing thermal energy more electrons arrive from the valence bond
or, since the cuticle is doped with various metallic impurities (Ishay et al.,
1982), electrons are donated to the conduction band until depletion occurs and
after a while (depending on previous conditions of the cuticle, the temperature,
the relative humidity and probably the composition of gasses as well as other
conditions not known yet) the conductivity diminishes. In cooling as well, there
is a decrease of the conductivity owing to the fact that fewer electrons receive
the energy needed to jump to the conduction band (MacDonald, 1962). The optimal
conductivity is at 28 ░C -29 ░C which is also the optimal temperature of the
hornet nest everywhere (Ishay and Ruttner, 1971; Heinrich, 1981).
Interestingly, at temperatures above optimal, that is above 28 ░C, the conductivity
starts dropping. The slope of this decrease varies from the slope of illumination
or that for cooling and all of them vary from the slope of warming, meaning
that there is a hysteresis between each of them. This phenomenon was noticed
earlier when measuring the resistance values upon warming versus those values
upon cooling the cuticle (Rosenzweig et al.,1985) and is a known feature
in other organic ferroelectric materials like thiourea or nucleic acids (Gutmann
and Lyons, 1981; Gutmann et al., 1983). The reason for the drop in the current
level, when warming beyond 28 ░C is probably phonon drag and/ or phonon scattering
(MacDonald, 1962; Ibach and Lüth, 1991).
In another study (Ishay et al., 1997), we found that exposure of a part
of the cuticle to light caused a sharp increase in voltage, when measured between
the illuminated and the dark part of the cuticle. The direction of this voltage
was reversed when the other part of the cuticle was illuminated. This voltage
was found to be linearly dependent on the intensity of the incident light for
relatively low light intensities of a few mW/ cm2. However, this lightinduced
voltage was much higher if the light beam was directed at the back part of the
cuticle strip than in the case where the front part of the cuticle strip was
illuminated by the same light beam. The spectral dependence of this effect was
also investigated and the maximum of the relative quantum efficiency was found
in the spectral range of 360-380 nm. It appears, that the cuticle might act
as a biological solar cell. From the foregoing, it appears that hornet cuticle
amounts to a photovoltaic cell which releases electric current in dependence
upon the temperature. The accumulated energy serves partly to maintain homeostasis
of the hornet's temperature by way of a Seebeck effect.
How is the cuticular photovoltaic system built-up ?
The system is comprised of an air sac acting as a bellows and of primary and
secondary tracheal ducts which pass along a series of photoreceptors and wind
around the base of each photoreceptor to form individual tracheal loops. The
respiratory rate changes in accordance with the ambient temperature and the
physiological needs, so that within narrow limits, efficient thermoregulation
is enabled by the conduction of air at the appropriate temperature. The temperature
of the conducted air is determined in situ, by a process whereby accumulating
electric energy in the cuticle is converted to thermal energy by a p-n junction
system. Additionally, the membrane around the tracheae contains openings through
which a product of an olfactory gland is evaporated, that apparently serves
as a thermoregulatory pheromone. In temperatures below optimal the adult hornets
(and wasps) commence to blow hot air around the developing brood (pupae) and
thereby warm it to the desired temperature (Ishay and Ruttner, 1971), whereas
when the temperature is higher than optimal, the adult hornets within the nest
commence to ventilate the brood or the entire nest (Ishay et al., 1967;
Sadeh et al., 1977). Thus, while the air sacs do contain the greater
share of the air supply, the temperature of the air is determined according
to need by its passage through the tracheal loops which gird the envelope of
the photoreceptor. Here, each tracheal loop comes in contact with: 1) the cuticular
envelopes of the photoreceptor Ñ they are electrically of n type (i.
e., electron donors) and 2) the yellow granules which are of p type (i. e.,
electron acceptors). The flow of air passing through the tracheal loop is thus
exposed to this p-n junction where electric energy is stored, or caused to flow
and this electric energy is transformed, according to need, into thermal energy
which is utilized in thermoelectric circuits such as have been described originally
by Seebeck and Peltier.
This process is geared primarily to provide thermoregulation of areas of the
abdominal cuticle or other areas that contain extraretinal photoreceptors since
it is crucial to keep the photoreceptors from overheating. The thermoregulatory
activity is important also for the entire nest, just as in vertebrates the excess
heat produced in the striated muscles and the liver is transported via the circulation
to all parts of the body.
Additional information about the photovoltaic system
In our subsequent studies the structure of the cuticle as a photovoltaic
cell was dealt with in greater detail (Ishay et al., 1992). Thus, we
have provided some information about the structure of the hornet cuticle in
the region of the yellow strips that are known already as a semiconductor-like
material (Ishay and Croitoru, 1978). The upper portion of the epicuticle is
flat and continuous, barring the region of the pores. As for the exocuticle,
it has vertical structures, namely, trabeculae, which provide mechanical support.
There are 30 or more parallel layers rolled around the abdomen, whose general
shape from below resembles a cone. These layers which are transparent or translucent
extend down to the region of the yellow pigment granules. The upper part of
the abdomen is convex, producing a lenticular shape that focuses the irradiated
light on the inner, yellow pigment granules, i. e., similar to a 'Fresnel lens'
(Maycock and Stirewalt, 1981). The voltage accumulates in the lower parallel
lamellae whence it is transmitted to the walls of the pore canals. These walls
descend to below the yellow pigment layer whose granules absorb all visible
light, except the wavelength of yellow (that is reflected) so that, most probably,
underneath there is darkness. The thicker (upper) layers of the cuticle close
off in the bottom part of the pore canal, while the thinner layers beyond the
closure point reexpand and at an angle of 90░ form thin plates of the hypocuticle,
sealing off the 'sandwich' from below. This photovoltaic system is active in
daytime for most of the hornets' lifetime, i. e., for workers several months
during the warm seasons, while for the queens a whole year. The 'sandwich' proper
is comprised of about 30 (or more) horizontal layers that are doped with Si,
P, S, Cl, K and Ca and smaller amounts of Mg, Fe and Zn, some of them electron
donors and others electron acceptors. The parallel layers progressively attenuate
from the exterior down to the yellow pigment layer, which is likewise horizontal.
In the vicinity of the pore canal, however, all layers become vertical and contribute
to the formation of the walls of the pore apparatus. The multilayer walls are
built as biological mirrors, i. e., they reflect the incident light due to their
optical thickness of about one-quarter of the wavelength of light (Land, 1972,
1985). This mechanism protects the content, i. e., the photoreceptor from overheating,
and so also the whole insect body. The pore apparatus includes the pore canal,
whose hollow portion serves as a light guide for the photoreceptor (Goldstein
and Ishay, 1996), while its walls conduct the electric energy formed in the
illuminated portion to the bottom, darkened part of the photoreceptor. The latter
attenuates into nippleshape in the region of the darkened hypocuticle. Here,
the electrical energy is transformed either into a current transmitted to the
hypocuticle plates or into a combined voltage which is transmitted, inter alia,
to the nerves that support the pore. In the dark, the electric resistance, which
in light was at a level of giga ohms (GW) drops down to a level of kilo ohms
(KW) Ñ a decrease of about 5-6 orders of magnitude (Ishay and Litinetsky,
1996). This difference prevents electrical current from flowing back into the
photovoltaic cells, i. e., in this respect behaving like a diode (Ben-Shalom
and Ishay, 1989). The dielectric fluid permeating all the internal spaces is
the hornet's hemolymph, which is transparent and of a yellow coloration (like
that of the yellow granules). The hemolymph of Vespa orientalis adults
has a pH lower than 7.0, i. e., is acidic; the osmolality range is between 321-593
mOsmole/ kg and the specific gravity is 1.022-1.028 (Joshua et al.,
1973). However, in cases of damage to the cuticle, the hemolymph darkens
Ñ oxidizes, thereby preventing the transmission of light (Whit-comb et
al., 1974; Ishay et al., 1997). It follows, then, that hornet cuticle
constitutes a combined system which integrates both photovoltaic and thermoelectric
elements, and it is this system which regulates the microenvironment both of
the sensory cells and their contained photoreceptors as well as of the nest
cavity and the contained hornet population. The mentioned conditions are essential
for proper development of the entire hornet colony. Thus, an appropriate temperature
is crucial for vespan sensing of gravity inasmuch as hornets build their nest
in the direction of the earth's gravitational pull (Ishay and Sadeh, 1975, 1977).
Sensitivity to gravity
The sensitivity to gravity has been examined in a number of studies.
By their behavior at the start of building, it is clear that hornets display
negative geotropism; however, the capacity to distinguish the highest geotropic
point, i. e., the zenith which is exactly opposite the center of gravity, is
probably not fully developed at eclosion but rather improves with time during
the first 3 days of life. At eclosion, an inclination of 5░ is sufficient to
cause the hornets' to start building at the point of inclination as if it were
the highest point of the container. It is only after several days that the hornets
are able to discern the highest point in any of the variously designed containers
(Ishay, 1976). Hornet workers, queens and males, aged 0- 24 hours (i. e. juveniles)
and 24 hours and more (i. e. adults) were tested by us for their responses to
changes in the direction of the gravitational force while placed on a flat surface
gradually tilted between 0.5░ and 180░ . The tests were run on non-blind and
blind hornets, at temperatures ranging between 18 ░C and 35 ░C, in daylight
as well as in the dark. Up to 18 hours of age, negative phototaxis prevailed
among the hornets, which displayed a clear preference for remaining in the dark
regardless of the geotropic position. Between 18-24 hours of age, there was
gradual appearance of a sensitivity to change in the geotropic position. Beyond
24 hr of age, the hornets became sensitive to changes in their declinations,
with workers becoming sensitive at a 3-5░ declination, queens at 4-5░ and males
at a declination of 8-19░ from the horizontal. Hornet response takes the form
of an upward climb, to the highest point of the test surface. Such response
required a temperature exceeding 24.8-25 ░C for workers, 23.2 ░C for queens
and 20.8-21 ░C for males (Ishay et al.,1986). It was found that as a
group, the hornets respond even to a 1░ inclination, but singly, the (maximal)
sensitivity or response is only to an inclination of 3-5░ . The hornets can
build a comb (oriented towards the gravitational force) when their multifaceted
eyes are covered; in fact the normal building activity is undertaken in the
dark and even by hornets that had been blinded or had eclosed in the dark and
had never seen any light. If part of the frons plate of hornets is damaged,
there is no building whatsoever, or the building is meager and the comb direction
is distorted. In other studies we investigated the sensitivity of hornets to
gravitation also under conditions of hypergravitation, created on a specially
designed centrifuge. We found that comb construction by hornets exposed to centrifugation
at 1 to 2 days of age differed from that of hornets similarly exposed at 3 to
7 days of age. Juvenile hornets built their cells in the direction of the resultant
force, whereas adults resisted the centrifugal force and tried to build in the
direction of gravitational force (Ishay and Sadeh, 1975). Furthermore, hornets
eclosing from and developing in combs subjected to centrifugal spinning, built
combs whose direction was affected both by rotation and by the resultant of
the gravitational and centrifugal forces. In all instances of building, whether
new, or restoration of the old, there was a relatively large deviation from
the direction of the computed resultant. However, when the hornets were removed
from the centrifuge they proceeded to build correctly, that is, in the direction
of the earth's gravitational force regardless of their previous environmental
conditions (Ishay et al., 1989).
The Ishay organ and its sensory epithelium -hair cell structures
In subsequent studies we found that the high sensitivity of the hornet
to directional changes in the gravitational force under differing of conditions
is enabled by the presence of a complex sense organ sensitive to varying accelerations.
This is the Ishay organ, which is located in the space between the frons plate
and the anterior part of the cerebroganglion. In this region are also located
the compound eyes (on both sides), the three ocelli (in the upper region) and
a pair of antennae (in the lower region). In addition, the region is traversed
at its base by nerves from the salivary glands and on both sides by muscles,
the largest of which are the adductor mandibularis, the lateral pharyngealis,
and the antennal muscles. While exploring the interior of the frons plate in
hornets and focusing on the structure of the conus, which intrudes inward from
the sutura coronalis, we detected in the various layers overlying one another,
yellow granules, stereocilia, bobs, and disclike plates. The latter proceeding
into the space of the acoustic box. The mentioned configurations are capable
of some mobility and are thus not strictly statoliths. The acoustic box is a
very complex organ with weighted bobs of various configurations that may aid
in gravity detection. Fibers and bobs within the acoustic box are immersed within
hemolymph which is enclosed by epithelium that may be piezoelectric. In insects,
the acoustic box may serve a similar function to what mechano receptors serve
in other invertebrates. The two components that can detect gravity are: (a)
the external sensors of the head and especially those on the frons plate which
are dry and static, and (b) those inside the acoustic box that are immersed
in hemolymph. Finally, the fibers and bobs inside the box may also serve a mechanoreceptive
function (Ishay et al., 1996). In further studies, we focused on the morphology
of the cilia which comprise the sensory epithelial element in the Ishay Organ
(see below). To date three types of hair cell configurations with stereoand
kinocilia have been described by us in the head of the hornet; these were encountered
at the vertex and frons regions adjacent to the three ocelli and are assumed
to be part of the hornet's gravity detecting system. The first and most
common type of hair cell configuration (type A) was a cell surrounded
by a septum, having a diameter of 30-50 Ám. Aggregates, of over 20 such hair
cell groups together, formed a larger unit, 130-300 Ám in diameter, which was
also enclosed by a septum. Many of these larger round units were, in turn, arranged
in either angular or leaf-like clusters. The hair cells bore numerous cilia
of 4.5-6.0 Ám long, and were themselves composed of smaller subunits of about
7-8 Ám in diameter, which were not enclosed by a septum.
The second type of hair cell configuration (type B) was made
of discrete cells with a diameter of approximately 12.5-14 Ám, located in the
vicinity of the pore canal outlet of the peripheral photoreceptor. These single
hair cells were either devoid of or only partially enclosed by a septum. Their
cilia were 4.5-6.0 Ám long as well, but with a diameter of only 150-160 nm.
On the exterior of each cilium a tubular system could be detected. Furthermore,
the tips of adjacent cilia were interconnected by a kind of fiber, bearing a
sperical body in its middle. The third type of hair cell (type C),
present in the neighborhood of the second type of hair cell (type B), was
chalice-shaped and had interconnecting fibrils comparable to those found at
type B as well. We believe, that these 3 types of hair cell configuration along
with the ganglion cells interconnecting their bases, are all components of the
gravity organ of the hornet, the Ishay Organ, and together with the cuticular
photoreceptors play a role in the navigation system of the hornet. We further
conjecture that the described structures are engulfed by endolymph and that
signals produced by each unit are conducted by neural fibrils to the hornet's
central nervous system (Jongebloed et al., 1999).
Graviception is important, also in vespan orientation during flight. The workers
in the nest of the Oriental hornet depart the nest for the field in foraging
flights that may take them as much as 5 km from the nest. Yet, even from such
great distances they are able to navigate their way back in great precision.
We believe the hornet's ocelli are contributory here. These ocelli are situated
on the vertex plate in such a fashion that if we draw a straight line through
each, we end up with an equilateral triangle. Each ocellus bears a convex cornea
shaped as a hemisphere. The tangential planes of each ocellus create between
them a pyramid of three equal sides. The arrangement of each ocellus on a different
plane enables a panoramic visual coverage of practically all the 360░ field
of vision above and around the head. Assuming that these ocelli pick up polarized
light, we surmise that hornets can sense the direction of the sun rays through
them. However, to be able to determine the direction of the sun vis-a-vis the
zenith, the hornet needs to orient itself in a position which is absolutely
horizontal with respect to the earth's surface or, alternatively, with respect
to hypothetical plane which is tangential to the earth's surface (Rosenzweig
et al., 1999).
References
-Ben-Shalom, A. and Ishay, J. S. 1989. The hornet cuticle as a diode
and an electric source. Phys. Chem. Physics, Med. NMR. 21( 1): 5-106.
-Edwards, R. 1980. Social Wasps. The Rentokil Library, Rentokil Limited, East
Grinstead.
-Goldstein, O. and Ishay, J. S. 1996. Morphology of a putative new peripheral
photoreceptor in social wasps. Phys. Chem. Physics, Med. NMR. 28( 4): 55-266.
-Guiglia, D. 1972. Les Gułpes Sociales (Hymenoptera, Vespidae) d'Europe Occidentale
et Septentrio-nale. Masson et Cie, Eds, Paris.
-Gutmann, F. and Lyons, L. E. 1981. Organic Semiconductos. Part A. R. E. Krieger
Pub. Comp. Malabar, Florida.
-Gutmann, F., Keyzer, H., Lyons, L. E. and Somoano, R. B. 1983. Organic Semiconductors
Part B. R. E. Krieger Pub. Comp. Malabar, Florida.
-Heinrich, B. 1981. Insect Thermoregulation. John Wiley and Sons, New York.
-Ibach, H. and Lüth, H. 1991. Solid State Physics, Springer, Berlin.
-Inokuchi, H. and Akamatu, H. 1967. Electrical conductivity in organic semiconductors.
Solid State Physics, 12: 93-140.
-Ishay, J. 1967. Observations on the behavior of the different members of a
colony of the Oriental hornet Vespa orientalis L. Ph. D. thesis, Hebrew Univ.
Jerusalem.
-Ishay, J. 1973. The influence of cooling and queen pheromone on cell building
and nest architecture by V. orientalis (Hymenoptera, Vespinae). Insectes Sociaux
70( 3): 243-252.
-Ishay, J. 1976. Comb building by the Oriental hornet Vespa orientalis. Anim.
Behav. 24( 1): 72-83.
-Ishay, J. and Croitoru, N, 1978 Photoelectric properties of the "yellow strips"
of social wasps. Experientia 34 (3): 340-342.
-Ishay, J. S. and Litinetsky, L. 1996. Thermoelectric current in hornet cuticle:
Morphological and electrical changes induced by temperature and light. Physiol.
Chem. Physics Med. NMR., 28: 55-67.
-Ishay, J. and Ruttner, F. 1971. Die thermoregulation im Hornisennest. Z. v.
Physiol. 72: 423-434.
-Ishay, J. S. and Shimony, (Benshalom) T. 1983. Electrical resistivity in cuticle
of Oriental hornet queen before, during and after hibernation : Evidence for
electronic conductance. Phys. Chem. Physics Med. NMR., 15( 4): 289-310.
-Ishay, J. and Sadeh, D. 1975. Direction finding of hornets under gravitational
and centrifugal forces. Science 190 (4216): 802-804.
-Ishay, J. and Sadeh, D. 1977. Geotropism of hornet comb construction under
persistent acceleration. Behav. Ecol. and Sociobiology, 2: 119-129.
-Ishay, J. S., Perna, B., Hochberg, Y. and Goldstein (Asanta) M. 1980. Photoelectric
properties of the yellow strips in Vespa orientalis : A mathematical model.
Bull. Math. Biol. 42( 5): 681-689.
-Ishay, J. S., Shimony (Benshalom), T., Lereah, Y. and Duby, T. 1982. Temperature
dependence of electrical resistance of hornet and ant cuticle in low temperature.
Direct current measurement. Phys. Chem. Physics, Med. NMR., 14: 343-361.
-Ishay, J. S., Fucks, C. and Rosenzweig, E. 1985. Temperature dependence of
the electrical resistance of hornet cuticle. A statistical model. J. Therm.
Biol. 10( 3): 137-144.
-Ishay, J. S., Shimony (Benshalom), T. and Arcan, L. 1986. The biomineralization
in Social Wasps (Vespinae): The presence of statoliths. Scan. Elect. Micros.
IV: 1619-1634.
-Ishay, J. S., Benshalom-Shimony, T., Weiss, D. and Kristianpoller, N. 1987.
Luminescence properties of the Oriental hornet Vespa orientalis. J. Luminescence
40& 41: 221-222.
-Ishay, J. S., Rosenzweig, E., Rosenzweig, O. and Berke, S. 1989. Geotropic
sensitivy of hornets. Adv. Space Res. 9 (11) : :147-155.
-Ishay, J. S., Benshalom-Shimony, T., Ben-Shalom, A. and Kristianpoller, N.
1992. Photovoltaic effects in the Oriental hornet. J. Insect Physiol. 38 (1):
37-48.
-Ishay, J. S., Pertsis, V. and Levtov, E. 1994. Duration of hornet sleep induced
by ether anesthesia is curtailed by exposure to sun or UV irradiation. Experientia
50( 8): 737-741.
-Ishay, J. S., Landsberg, A. and Pelah, S. 1996. Micromorphology of the fibers
behind the frons plate and its adjacent regions in the Oriental hornet (Hymenoptera,
Vespinae) Scann. Microsc. 10( 1): 187-208.
-Ishay, J. S., Rosenzweig, E. and Solomon, A. 1997. Thermoregulation of the
extraretinal photoreceptor apparatus in the yellow stripes of the gaster of
hornets. Phys. Chem. Physics Med. NMR., 29 (2): 213-230.
-Jongebloed, W. L., Rosenzweig, E., Kalicharan, D., J. J. L. van der Want and
Ishay, J. S. 1999. Ciliary hair cells and cuticular photoreceptors of the hornet
Vespa orientalis as components of a gravity detecting system: a SEM/ TEM investigation.
J. Electron Microsc. 48 (1): 63-75.
-Joshua, H., Fischl, J., Henig, E., Ishay, J. and Gitter, S. 1973. Cytological,
biochemical and bacteriolo-gical properties of hemolymph and other body fluids
of Vespa orientalis. Comp. Biochem. Physiol. 45B: 167-175.
-Land, M. F. 1972. The physics and biology of animal reflector. Progr. Bio.
Biophys. Mol. Biol. 24: 75-106.
-Land, M. F. 1985. Optics of insect eyes, In: Comprehensive Insect Physiology,
Biochemistry and Pharmacology, Vol. 6 (G. A. Kerkut and G. J. Gilbert, eds.),
pp. 225-275.
-MacDonald, D. K. C. 1962. Thermoelectricity: An introduction to the principles.
John Wiley & Sons Inc., New York.
-Matsuura, M. and Yamane, S. 1990. Biology of the vespine wasps. Springer-Verlag,
Berlin.
-Meier, H. 1974. Dark and Photoconductivity of Organic Solids. Verlag Chemie,
Bamberg.
-Maycock, P. D. and Stirewalt, E. N. 1981. Photovoltaics. Sunlight to Electricity
in One Step. Brick House Pub. Co. Andover, MA.
-Rosenzweig, E., Fucks, C. and Ishay, J. S. 1985. Electrical resistance of hornet
cuticle: Changes induced by Xanthines Ñ A statistical model. Phys. Chem.
Physics, Med. NMR., 17 (4): 435-449.
-Rosenzweig, E., Solomon, A. S., Kirshboim, S, Ishay, J. S., Want, H. van der,
Kalicharan, D. and Jongebloed W. L. 1998. The ocelli in Vespa orientalis : Micromorphology
and function. Phys. Chem. And Physics and Med. NMR 30, suppl.: 241-269.
-Sachse, H. B. 1975. Semiconducting Temperature Sensors and their Applications.
John Wiley and Sons, New York.
-Sadeh, D., Ishay, J. and Yotam, R. 1977. Hornet ventilation noise: Rhythm and
energy content. Experientia 33( 3): 335-377.
-Shimony, (Benshalom) T. and Ishay, J. S. 1984. Electrical capacitance in hornet
integument: Frequency, light, and temperature dependence: possible p-n junction
effect. Physiol. Chem. Physics Med. NMR., 16 (4): 333.
-Spradbery, J. P. 1973. Wasps. Sidgwick and Jackson, London.
-Whitcomb, R. F., Shapiro, M. and Granados, R. R. 1974. Insect defense mechanisms
against microorga-nisms and parasitoids. In: The Physiology of Insecta, 2nd
Edition (M. Rockstein, ed.), Vol. V, Academic Press, New York, pp. 447-536.
-Wilson, E. O. 1971. The Insect Societies. Belknap, Harvard, Mass.