When the specimen is charged with an electrical current under fixed temperature,
the current attains several nAmp in darkness, however is usually less than that
under illumination by an order of magnitude. The capacitance values range between
1-7 mFarad both in light and in the dark. At a temperature of 4 °C or thereabout
the cuticle undergoes "relaxation" and becomes charged. This occurs in the dark,
for at least several hours, whereas transfer of the specimen to a higher temperature
yields a discharge current of 30- 40 nAmp, for two hours. Moreover, it is vital
for the relative humidity to be high (about 90- 99 %) thus enabling the episodes
of charge and discharge to be reversible, i. e., that the cuticle does not "tire"
(Ishay et al, 1995).
The manner in which the cuticle undergoes "relaxation" during cooling in the
dark and subsequent rise in the discharge current upon gradual warming in the
dark qualifies it to be named a material endowed with thermoelectric properties
(Egli, 1960). From these studies it was pointed out that the cuticle acts like
a photovoltaic system (Ishay et al, 1992) and that it exhibits a thermoelectric
effect (Shimony and Ishay, 1981). The cuticle behaves like an organic semiconductor
with traps (Ishay et al, 1980b).
Thermoregulation system of the extraretinal photoreceptor apparatus
An important feature in the thermoregulation system is that of the extraretinal
photoreceptor apparatus. We have directed our study in particular on the photoreceptor
apparatus in the yellow strips of the gaster of hornets. The system is comprised
of an air sac acting as a bellows and of primary and secondary tracheal ducts
which passes along a series of photoreceptors and wind around the base of each
photoreceptor to form individual tracheal loops. The respiratory rate changes
in accordance with the ambiant temperature and the physiological needs, so that
within narrow limits, efficient thermoregulation is enabled by the conduction
of air at the appropriate temperature. The temperature of the conducted air
is determined by an in situ process whereby accumulating electric energy is
converted to to thermal energy by a p-n junction system (the Seebeck effect).
Additionally, the membrane around the trachea contains openings through which
a product of an olfactory gland is evaporated, that apparently serves as a thermoregulatory
pheromone.
The Oriental hornet breathes via trachea (Snodgrass, 1925; Duncan, 1939, Imms,
1960). The gaster segments of hornets each contain a pair of spiracles on their
tergites plates. However, these openings of the tracheal system are clearly
visible only on segments II and III of the hornet, whereas on the other segments
they can only be discerned when the abdominal segments are at full stretch (Spradbery,
1973). As mentioned before, the spiracles admit air into the tracheal ducts
as well as into the dorsal and ventral air sacs which extend transversely in
the gaster. Wigglesworth (1963) lists a number of roles attributed to these
air sacs, including the ventilation of muscles in the course of flight of the
insect.
For some time now we have been studying with the field emission scanning electron
microscopy and transmission electron microscopy, the micromorphology (Goldstein
and Ishay, 1996) and the physiology (Goldstein et al, 1996) of photoreceptors
located in the gaster region of the hornet on the cuticular areas containing
yellow pigment (Ishay et al., 1994; Kristianpoller et al., 1995; Ishay et al.,
1997).
Consequently, we deemed it worthwhile to ascertain the manner in which thermoregulation
is effected in such parts of the cuticle which contain extraretinal photoreceptors
Ñ the socalled yellow stripes. Indeed, we identified morphologically
in these regions peripheral photoreceptors and these were encountered in all
the species of social wasps (Vespinae and Polistinae) which fly out of the nest
in daytime for their daily needs (Goldstein and Ishay, 1996).
Details on the biology of social hornets and wasps have been published extensively
in the last 60 years (Duncan, 1939; Ishay et al., 1967; Wilson, 1971; Guiglia,
1972; Spradbery, 1973; Edwards, 1980; Matsuura and Yamane, 1990; Ugotini and
Cannicci, 1996). In a previous study, one of the authors (JS Ishay) investigated
the respiratory rate during various daily activities of the hornet V. crabro
which is prevalent throughout the northern hemisphere. Ishay found that while
standing outside the nest this hornet breathes 40-80 times a minute (compared
to a much slower respiratory rate inside the nest), when drinking water it respires
100-135 times per minute and while warming the pupal brood, between 160-210
times a minute. It stood to reason that such marked differences in respiratory
rate must be connected both with thermoregulation as well as with supplying
oxygen to the tissues. As for the former, the question arose as to how the vespan
respiration acted to regulate the temperature within the cuticle on the one
hand and in its ambiance of the nest in general, on the other. The present chapter
attempts to provide an answer, albeit partial, to some of these queries.
Materials and Methods
Specimens of V. orientalis were collected from natural nests
in the field in Israel, while specimens of V. crabro were collected in
Frankfurt, Germany, as described elsewhere (Ishay, 1964). Specimens to be tested
were anesthetized by diethyl ether and prepared, while narcotized for observation
and photography through a light microscope and a Scanning Electron Microscope
FE-SEM as well as by a method previously described, using small strips of freshly
collected homers (Ishay and Ganor, 1992). It is worth pointing out that lightmicroscopy
enables one to see the photoreceptors on the inner side of the cuticle and also
a little of the morphology of surrounding structures, be they beneath the basal
membrane and under the hypocuticle, whereas FE-SEM does not enable such viewing
so long as the overlying hypocuticle is intact.
Results
Figure 1 shows two hornets of V. orientalis: on the left one
with an abdomen of ordinary length and next to it, for comparison purposes,
a queen with an expanded abdomen (gaster). In the latter, one notes that between
the gastral segments with yellow pigment there appears a cuticular stripe of
a brown color. In a hornet with an abdomen in the normal (contracted) state,
such a brown stripe (and a connection membrane) is masked by the yellow stripe
of the preceding segment because the distal stripes of yellow cuticle on the
gaster usually protrude beyond the segment to overlie the surface of the next
segment. All the gastral segments are arranged like a telescope that can be
artificially lengthened, either by pulling on the extremities as described in
Figure 1 (left) or naturally during the process of respiration. This abdominal
extension is enabled owing to the fact that between each two successive segments
there is a connecting membrane which has been named the intersegmental conjunctive
(ic) (Duncan, 1939). This IC is elastic so that during rest, in the relaxed
state, the one segment forms an eave which overlies the segment behind it. In
this way we get "an extensive reduplication." "This strengthens the tergurn
and provides a sclerotized bearing to glide over the base of the succeeding
segment" (Duncan, 1939). This is also the area where parasite insects of the
strepsiptera stylopide (i. e., parasitize). As adults they 'sit' on the dorsal
site of the abdomen between the sclerites (Krombein, 1967). In our case, in
gastral segments 4 and 5, the part of the segment schematically demonstrated
in Figure 2, that produces the reduplication is invariably that containing the
yellow pigment. In this 'yellow' region both in the dorsal and ventral surfaces
of the cuticle, there are two brown spots (I in Figure 2). If we evert the segment
and inspect its interior surface (i. e., the surface facing the body), we note
that from the air sac which extends across the brown region of the segment (2
in Figure 2) emerge tracheal ducts that proceed in the direction of the yellow
stripe; these ducts emerge either individually from the central region of the
air sac (3 in Figure 2) or, from each lateral end of the air sac. These emerge
as a braid of tracheae that terminates in the region of the brown spot on the
inner side of the yellow cuticle (4 in Fig ure 2). A similar pattern was displayed
also on the inner, ventral side of the cuticle. The tracheae comprising the
'braid' split into thinner ducts which proceed beneath the basal membrane and
the hypocuticle. Here each tracheal duct passes across a 'line' of photoreceptors
(usually 8-15), to each of which it sends a tracheal 'loop', which circumvents
the ventral side of the photoreceptors (6 in Figure 2 represented as small circles).
The yellow stripe terminates in a tuft of setae (small hairs). The membrane
which interconnects the two brown spots (6 in Figure 2) represents a membrane,
which links the segment to the one in front (and in the intact hornet this would
be the underside). This intersegmental conjunctive is the IC and it actually
separates what is attached to the interior of the body. The upper part, above
the membrane is protruding outwards to the eave or reduplication. The IC membrane
thus bisects the yellow stripe approximately at the middle. Figure 3 provides
a schematic representation of the peripheral photoreceptor indicating the most
prominent structures and their relative distribution. The membranes of the air
sacs and tracheae in V. orientalis are shown in Figures 47. One such
ramification leading into a tracheae (1), the structure of the outer membrane
(2) and the structure of the inner membrane (3) is displayed in Figure 4. Figure
5 comprises a photograph (in part) of the inner membrane in an air sac with
longitudinal, hardened ribs (1) and the ridges surrounding the passage to the
trachea. Between the hardened ribs secondary ribs can be seen that apparently,
respond to tension. In Figure 6 we see a striped membrane in which some of the
bands are protruded and hardened. The bands are comprised of 2-3 secondary bands
extending to a length of 10mm or more, originating from a region at some remove
from the connection with the trachea. In all these three figures (4, 5 and 6),
one can discern, at various magnifications, membranes of the air sac. In segment
4 of the gaster: these membranes bear contractions that are typical for piezoelectric
membranes. Those respond to tension and pressure (mechanical stress) by producing
electricity (or electric polarity).

FIGURE 1. Two hornets are shown of which the one on the left has a contracted
gaster as is customary during rest. The specimen on the right has been intentionally
stretched in order to show firstly the difference in abdominal length in the
two conditions (extension and relaxation) and secondly to reveal the two brown
stripes which during relaxation are concealed underneath the other plates.
FIGURE 2. A scheme demonstrating structure of the cuticular
yellow stripes. On top can be seen a homet with yellow stripes (-x I). In each
of these stripes there are, both in the upper tergite and the bottom sternite,
two brown dots (or spots) (1). When one inverts such a yellow stripe and views
its interior surface under a microscope, one can see an air sac extending across
the brown part of the segment (2) from which emerge braided clusters of tracheae
that proceed to each of the two brown spots (4). From the same air sac arise
also more attenuated tracheal ducts (3) and similarly these ducts pass underneath
the BM and the hypocuticle to reach the lower part of the photoreceptors and
continue to the end of the stripe (5); these also loop around each photoreceptor.
The yellow stripe terminates with a line of dispersed hairs (not seen here).
Separating between the part protruding from the body and the part contained
within the body is a membrane (6). A branch of a trachea which surrounds the
base of a photoreceptor is shown (7) and the lower part of the photoreceptor
enlarged to join the hypocuticular layers (8). YG-yellow ganules; BM-basal mem-brane;
IC-intersegmental conjunctive; A-upper side of the cuticle (epicuticle); S-spiracle.
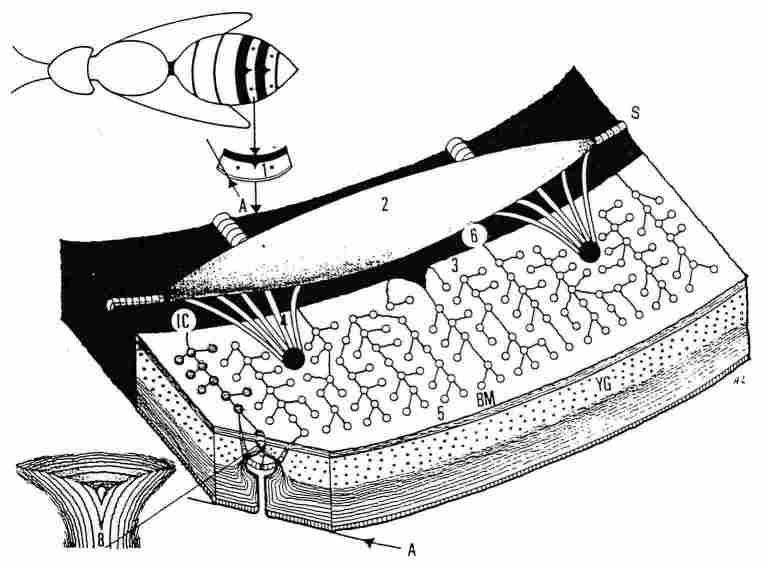
FIGURE 3. Schematic representation of the peripheral photoreceptor. 1) the epicuticle;
2) the reticular fibers at the periphery of the bulge; 3) the exocuticle; 4)
the axon that connects to the membrane of the bulge and the axonal microfibrils;
5) the gap junction of glial membranes; 6) the outer vitreous body; 7) the inner
vitreous body; 8) the annular trachea; 9) the granular area at the center of
the photoreceptor cell; 10) the microlamellae and their black edges; 11) the
nucleus of the PR cell; 12) a glial cell; 13) nucleus of glial cell; 14) basal
membrane cell; 15) cuticle under the basal membrane; 16) reticular tissue at
the lower end of the bulge; 17) the pigment granules at the periphery of the
PR cell 18) the cuticular lamellae.
Apparently, these portions of the membranes are capable of extending or contracting
as the need arises and according to the content of air in the air sac. A typical
trachea with annular hardenings which protrude from the inner surface of the
trachea the tenidia (1) is shown in Figure 7. Everywhere, small masses are present
which apparently contain aggregates of lipid cells. Presented in Figure 8 is
a view of the inner side of segment 4 in the gaster of the homet. Here "I" indicates
the surface of the region containing yellow pigment. Next, "T' indicates the
attachment point of the 'braid' of the tracheal ducts to the 'spot' of brown
cuticle. The interconnecting membrane which encircles this region belongs (and
connects) further on to the IC is situated more anteriorly to it. On the right
side of the figure, there is also the same 'braid' of tracheae, is covered and
therefore invisible. Yet the 'braid' connects with the air sac through thicker
ducts (4) that link it to the sac (5). Behind the IC one can discern an amorphic
mass (6) which is possibly a glandular excretion (see below). A somewhat more
enlarged view, of the tracheal 'braid' is shown in Figure 9, in which the following
landmarks are discernible: I ) the inner membrane of the brown stripe of the
segment (No. 4): 2) the membrane of the air sac with the annular bracings on
the inner side: 3) a 'braid' of tracheal ducts that interlink to the brown 'spot'
on the cuticle containing yellow pigment: 4) the epithelium of the IC: and 5)
the inner region of the yellow stripe. Further details of the air sac are shown
in Figure 10. Here, we can see: annular bracings (I) tracheal ducts interlinking
one sac to the other

FIGURE 4. The microstructure of the air-sac membranes at their juncture with
a tracheal duct. Noticeable are struc-ture of the membrane at the junction point
(1). structure of the external membrane (2) and of the internal one (3). Even
from this viewing. it is reasonable to suppose that these membranes are stretchable
when the air sac fills up and contractible when the air sac empties, whether
externally through the spiracles (S) in Figure 2) or internally into the tracheae
and tracheoles that carry air to the tissues and cells.
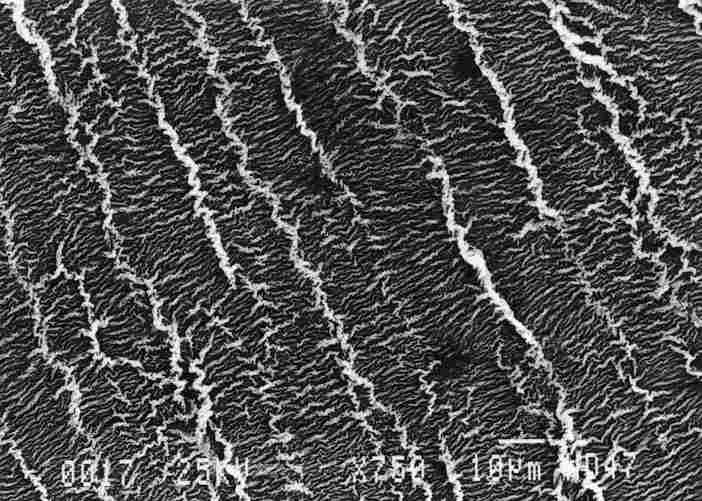
FIGURE 5. Girding the passage to the tracheal duct are a series of ridge I )
on the epithelium, with secondary ridges between them. resembling the slats
of a sunshade. These configurations are apparently typical for membranes that
undergo stretching and relaxation
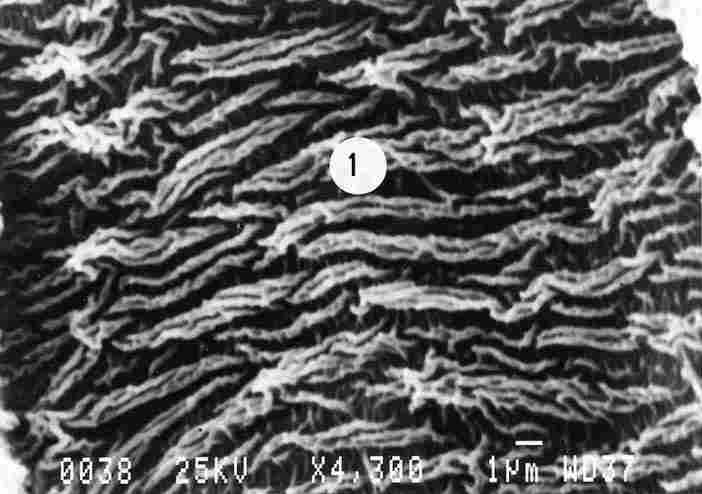
FIGURE 6. Prominent ridges are visible which are comprised of 2 or more stripes
(1). More prominent are those which are braided together. Each of the secondary
stripes that comprise the primary ridged stripe is shorter than the total stripe.
The membrane was photographed from a distance at some remove from the opening
of the tracheal duct. What we see are the ridges appearing intermittently.
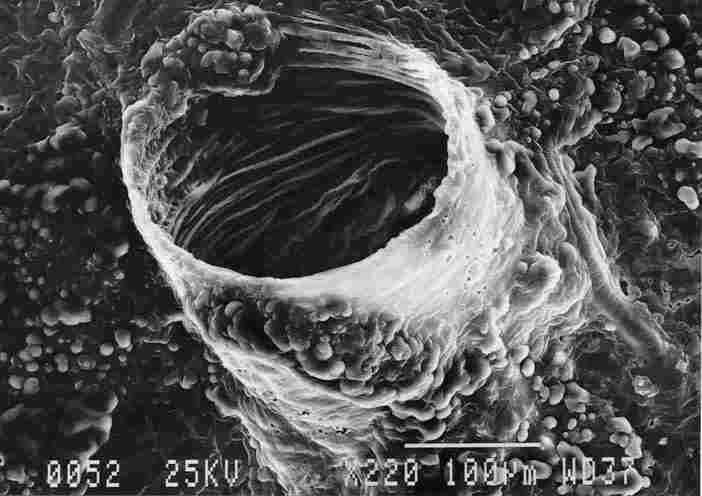
FIGURE 7. Typical tracheal outlet with annular ridges on the side facing the
tenidium (1). Everywhere nearby and on the exterior of the trachea can be seen
small protuberances (2) containing, apparently, aggregates of fat cells
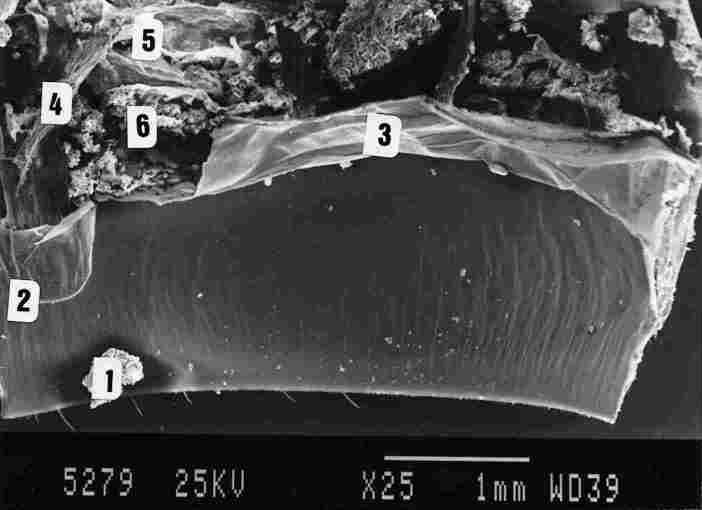
FIGURE 8. A SEM micrograph showing the inner surface of the yellow stripe (1),
the entry point of the tracheal 'braid' into the brown spot (2). The membrane
designated as intersegmental conjunctive (IQ (3). tracheal ducts entering the
air sac (4) and finally portions of the air sac (5). Note that a picture taken
by SEM does not enable viewing beneath the BM and hypocuticle and therefore
one cannot see the lower site of the photoreceptors, nor the tracheal loops
which envelop them. These structures can be seen through a light microscope.
Behind the IC are masses of an amorphic material (6). These may possibly represent
a gland that produces pheromones.
(2): bracing bands extending down the inner length of the sac (3); and finally
the supporting membrane across the tracheal ducts which proceed into the 'braid'
(4). The tracheae which emerge from the air sac gradually narrow into more delicate
ducts (1 in Figure 11) which receive support from transverse membrane inside
(2 in Figure 11). This supporting membrane is increased in size upto to the
lower portion of the tracheal duct 'braid' which is fully replete with transverse
supportive membrane. This membrane appears like a mesh or rete fastened on the
exterior of all ducts in the braid (see 1 in Figure 12). Underneath the membrane
(BM + hypoticle) are the tracheal ducts. At the point of entry to the brown
spot area (it looks like a basket), the "network " individually enveloping each
duct comes to an end. In the same region (Figure 13), the cuticle of brown spot
within the "yellow "area forms an elevation reminiscent of a stunted goblet
and this at the juncture point between the tracheae and the cuticle (which is
not seen in the picture); around and quaquaversal (that radiate from a central
mass) from the goblet (which height from the distal side of the cuticle is about
0.5 µm) extends the membrane called IC. This membrane which covers the tracheal
braid on the distal side is comprised of bands. Hence in Figure 13, we discern
the IC made of bands (10, the bottom of the tracheal duct braid (2), and the
tracheal ducts enveloped in a supportive membrane (3). An enlargement of the
membrane at the base of the tracheal duct braid is shown in Figure 14. One clearly
sees that the area is replete with bands, whose width is usually less than 10
µm and sometimes even as little as 5 µm, while on the underside of the bands
(2) there are "fringes" (like the fringes of a dress) of nonuniform length which
impinge upon the band underneath them. The bands are attenuated only in the
region abutting the tracheal 'braid' whereas outside this region, they are broader
(3).
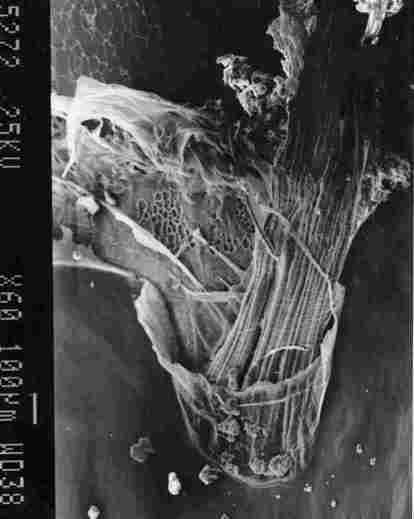
FIGURE 9. View of a tracheal 'braid'. One can see the inner membrane in the
brown stripe of segmemt IV (I). the membrane of an air sac with lacework bracings
on the inner side (2). a , "braid' of tracheal ducts (3) which connect with
the brown spot in the yellow stripe of' the segment. the IC membrane (4) and
the inner region of the yellow stripe (5).
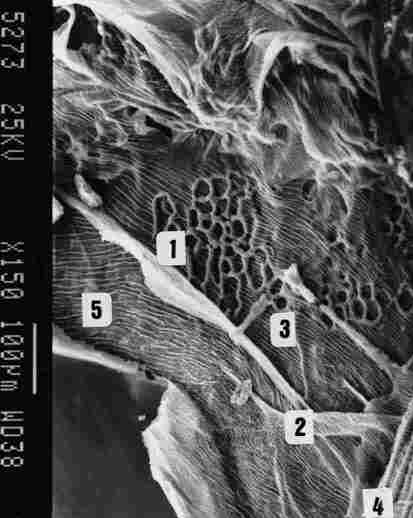
FIGURE 10. Further enlargement of the previous pic-ture, showing the latticed
bracings ( I ). tracheal ducts passing between two air sacs (2). supportive
ridges along, the inside of the sac (3). supportive membrane across the tracheal
ducts that form the braid (4) and finally the IC membrane (5).

FIGURE 11. The tracheal ducts which form the *braid*. In the top part of the
picture the ducts are thicker (I) but these gradually "split" into more delicate
ducts (2) At bottom can be seen a supportive netting that enwraps each cluster
of ducts.

FIGURE 12. This micrograph shows the supportive network ( I ) that envelops
the tracheal ducts that pas, from the air sac into the brown spot of the yellow
stripe.
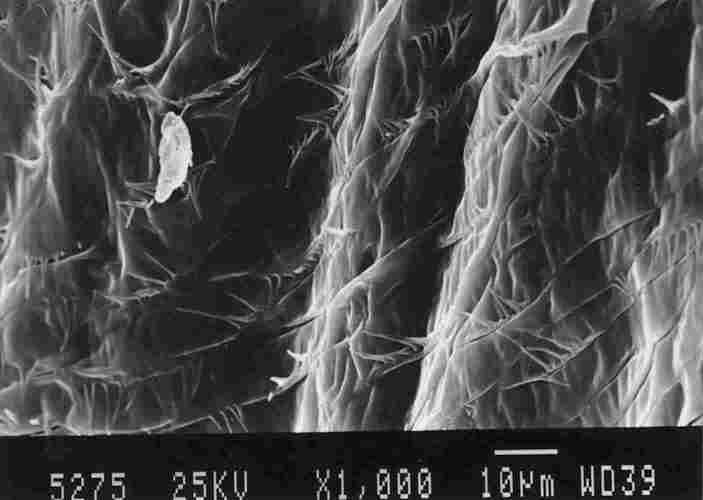
FIGURE 13. On the right can be seen a basket-like structure. This is a structure
that is part of the IC enveloping the tracheal 'braid'as it connects with the
brown spot on the yellow stripe ( I ). Above the basket-like structure one can
see the ends of' the tracheae (2) which are surrounded by a supportive network
(3), This network seems to be a part of and interlinked with the membrane of
the air sac (4). On close inspection. one notes that the IC membrane which makes
up the basket-like structure is composed of multiple bands (see below).
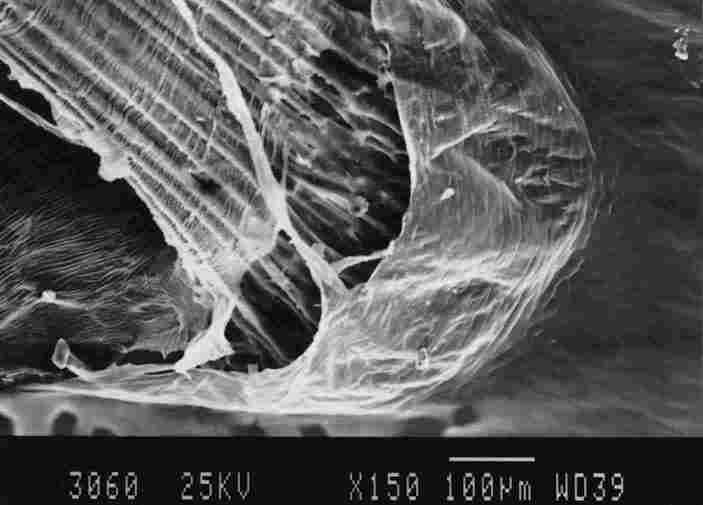
FIGURE 14. Enlargement of the membrane in the basket which is at the base of
a tracheal 'braid'. One notes that the membrane is multiple banded ( 1). each
hand 5-10 mm in width. there are gaps between each two bands (stripes) and "fringes"
are suspended from these bands to touch the underlying band. The "fringes" are
of variable length and between every group of "fringes" there are gaps (3).
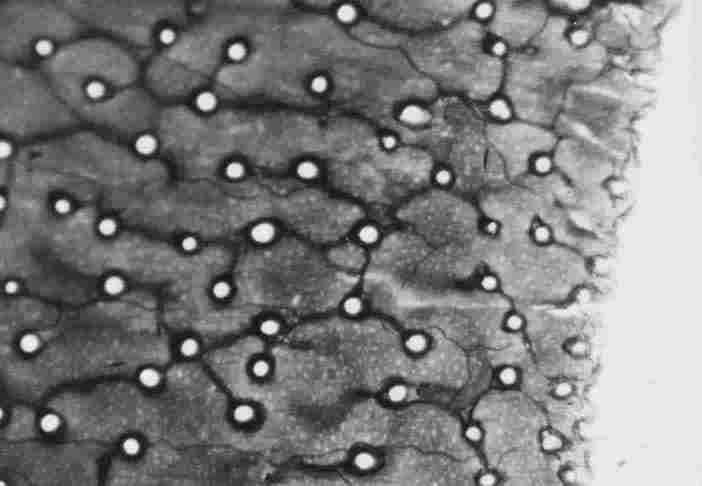
FIGURE 15. Enlargement of a number of bands and "fringes' that were shown in
the previous Figure. One can discern groups or fringes (4-12) of variable length
(arrow), all of which are touching the band underneath them. Between one group
of "fringes" and another, there is a gap (1) and there are slits of about I
mm between every two bands at the bases of the "fringe"( 2). The bands are concentric,
with intercalation, of other bands, so that there is no direct parallel continuity
of long bands.

FIGURE 16. Micrograph taken via light microscope of the tracheal duct( s)) passing
between the photoreceptors underneath the BM and hypocuticle. The light-colored
circles are the photoreceptors and the light reflecting through them from the
other (upper) end of the cuticle( 1) Also visible is the tracheal ducts passing
along a row of photorecep-tor (3). The magnification is x150. Bar= 100mm
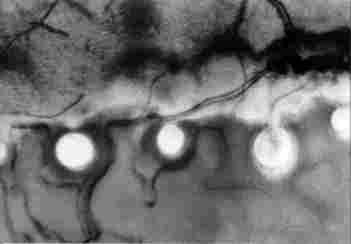
FIGURE 17. Enlargement of a portion of the previous figure. Hcrc, can be seen
a tracheal branch passing between the photoreceptors ( I ) and looping around
each one of them (2). Also visible are what appear to be nerve branches ( 3).
Magnification x400. Bar = 100 µm

FIGURE 18. After peeling off the BM and hypocuticle in the yellow stripe one
sees the base of the photoreceptor (1). the concentric rings of cuticle enveloping
the entire photoreceptor (2). the granules of yellow pigment (3) and the tracheal
loop that envelops the base of the photoreceptor (4). Note that the external
diameter ot the tracheal loop here is about 3 mm. We need to point out that
the specimen from which this picture was taken was the hornet V. crabro.
A larger magnification of these bands is given in Figure 15, where one can
see: (1) areas in which the bands are not separate throughout but only intermittently
so (1). (2) the number of "fringes" in each bundle is between 4-12. (3) the
gap between any two bands is smallabout 12 µm (2). (4) the "fringes" are of
nonuniform length usually between 1-10 µm. (5) in the same bundle there are
no two consecutive bands of the same length. All the "fringes" touch at various
places the lower band (arrow). Tracheae pass through the tracheal 'braid' and
then beneath the BM and the hypocuticle following which they traverse the yellow
cuticle and contribute a tracheal loop around the bottom of each photoreceptor.
All this is shown in Figure 16, which was taken via LM. Here we see clear circles
through which light reflects from the other (upper) side of the cuticle; these
are the photoreceptors. See also Figure 2 (6) and Figure 3 (4), this is the
lower end (the inner cuticle) (arrow at 1) with a tracheal loop around the circle
(arrow at 2) and also tracheal ducts that pass longitudinally, arrow at (3).
In Figure 17, taken through a LM at higher magnification (x 1000), one can see
a branching of the tracheae ( 1) which sends a tracheal loop to the base of
a photoreceptor (arrow at 2). In the region is discernible also a neural fibre
(3). Removal of the BM together with the hypocuticular layers reveals the lower
side of the photoreceptor, Figure 18 (1), with concentric rings of cuticle sealing
it there (2), granules of yellow pigment (3) and also part of the annular trachea
which girdles the base of the photoreceptor (4). This picture was taken from
the cuticle of V. crabro, whereas all the others are based on the cuticle
of V. orientalis.
Discussion
The system described in the present study contains: (a) A mechanism
that enables the passage of air in pipes, as in a thermoregulatory apparatus,
and is comprised in the case of hornets of air sacs of variable volume that
act as a bellows and of hardened tracheae which transport the air and split
into more slender and elastic branches that pass among the photoreceptors. Except
in the region of the spiracles, where the air can be admitted or expelled by
the opening or closure of these respiratory structures, the rest of the tracheal
system is a closed one. (b) The outlets of glands that release their evaporant
contents between the segments of the gaster. We conjecture that the described
system is geared primarily to push and compress air into the bases of the photoreceptors
so as to regulate the temperature there and if so, the rate of respiratory movement
is coordinated with physiologic activities of the hornet. The cooling or warming
accomplished via the transport of air within pipes is well known and widely
used in human technology. The process requires, of course: (1) thermosensors
that are sensitive to temperature so that any deviation from the desired temperature
will activate a corrective system. (2) the determination of a set point, that
is, the optimal temperature which needs to be an equilibrium between heat creation
and heat loss and (3) the ability to raise or lower, ad libitum, the temperature
of the air conducted through the pipes especially through those loops of tracheae
around the base of the photoreceptors. As for the latter setpoint, there is
solid evidence that the temperature in the nest of hornets (Vespinae) is constantly
set to 29 °C (Ishay et al., 1967; Ishay and Runner, 1971; Ishay, 1972; Ishay,
1973; Heinrich, 1981; Ishay and BarenholzPaniry, 1995). It may also be presumed
that thermoregulatory sensors are distributed throughout the hornet cuticle.
Additionally, there are tracheae throughout its length, while air sacs are arranged
transversely in the abdominal segments as well as elsewhere. The air sacs are
actively contractible and can thus compress the air in the system through the
use of muscles, following which they relax passively. The air sacs which are
arranged crosswise in the abdominal segments underneath the cuticle can also
act as cushions that glide between the partly superposed segments and thus prevent
excess friction between them during respiration. Judging strictly by their morphological
appearance, the membranes of the air sac appear to be piezoelectric, responding
to pressure by creating an electric voltage which helps to signal the physical
properties of the air. The air that flows in the regions of the yellow cuticle
provides oxygen to the local cells, as is to be expected, but the density of
the tracheae in these regions points to another, very important role, namely,
thermoregulation. Such thermoregulation results from the uptake of light in
the visible wavelengths and of heat and their conversion to electric energy,
whether as voltage (under illumination) or as current (in darkened, inner portion
of the cuticle or under conditions of darkness) and by analogy the cuticular
region resembles a solar cell (Maycock and Stirewalt, 1981; Ishay et al., 1982;
Ishay and Litinetsky, 1996; Ishay et al., 1997).
In view of the fact that the cuticular stripes possessing yellow pigment protrude
to the exterior of the body, it seems that maintenance of their proper function
is crucial, and this for the following reasons: (a) intensive or ordinary respiratory
activity persists even when the hornet is anesthetized (Ishay et al., 1994)
as well as when the abdomen (of any insect) is detached from the body (Huber,
1960; Miller, 1965; Farley et al., 1967), being regulated by the ventral ganglia,
both the thoracic and abdominal ones (Grass, 1976). (b) the concentration of
tracheae in a 'braid' which effectively provides tracheal rami to the yellow
stripes suggests an effort to ensure maximal yellow stripe surfaces that are
free from any interference by other tissues (and acting as thermoradiators).
(c) the creation of a tracheal loop around the base of each photoreceptor. We
still need to address the question as to how the air flowing through the tracheal
ducts acquires the desired temperature. At temperatures below optimal the adult
hornets (and wasps) commence to blow hot air around the developing brood (pupae)
and thereby warm it to the desired temperature (Ishay and Rutter, 1971), whereas
when the temperature is above optimal, the adult hornets within the nest commence
to ventilate the brood or the entire nest (Ishay et al., 1967; Sadeh et al.,
1977). Thus, while the air sacs do contain the greater share of the air supply,
the temperature of the air is determined according to need by its passage through
the tracheal loops, which gird the envelope of the photoreceptor. Here, each
tracheal loop comes in contact with 1) the cuticular envelopes of the photoreceptor,
they are electrically of n type (i. e., electron acceptors); and. 2) the yellow
granules, which are of p type (i. e., electron donors). Flow of air passing
through the tracheal loop is thus exposed to this p-n junction where electric
energy is stored, or caused to flow, and this electric energy is transformed,
according to need, into thermal energy, which is utilized in thermoelectric
circuits such as have been described originally by Seebeck and Peltier. This
process is geared primarily to provide thermoregulation of areas of the abdominal
cuticle or other areas that contain extraretinal photoreceptors, since it is
crucial to keep the photoreceptors from overheating. The thermoregulatory activity
is important also for the entire nest, just as in vertebrates the excess heat
produced in the striated muscles and the liver is transported via the circulation
to all parts of the body. It is known that in humans, the metabolic process
which takes place in the retina, needs high supply of oxygen and creates energy
excess. The high rate of blood flow through the uvea is very important by giving
high pO 2 in the uvea, which facilitates the diffusion of oxygen into the retina
and it also helps to protect the eye from thermal damage even under rather extreme
conditions, such as arctic snowstorms, Finish sauna bath, and observation of
very bright objects (Moses, 1975). We see in the air supply system in the Oriental
homet the exact analogue of the choroidal system in humans fulfilling the same
physiological functions and complementary to it a source of energy for its physical
activity. As for vaporization of volatile substances through the openings in
the membrane designated as IC near the brown spots in the yellow stripes, we
note that Spradbery (1973) points out that in sternite VI of hornet abdomen
a gland is located, which Vecht (1968) described as one that releases pheromones.
What we have described herein is probably a different gland, because its numerous
openings are located on gastral segment IV, and the gland itself is situated
on a tergite (a dorsal plate). So far as we know, no glands have been described
in the specified segment and ours is thus a first description. We are in the
dark yet as to the chemical nature of the substance evaporated from the described
gland, but the mere fact of being connected to the bellows system of the air
sacs with tracheae must enhance its efficiency. We have observed that when the
bellows increases its activity the gland releases its volatile contents at a
comparable rate and according to the intensity of respiration. It stands to
reason that the gland is associated with thermoregulation, perhaps also signaling,
altering and recruiting in emergencies, to the urgent need of cooling or heating
the brood. If this be true, then this is the venue of thermoregulatory pheromone
which has been speculated about years ago (Ishay, 1972; K"niger, 1977). Interestingly,
a structure comprised of fringes has previously been reported in termites (Caloterme
fiavicollis) by Lebrun (1971) in conjunction with a tergal gland in the
9th abdominal segment. The fringes in his and also in our case are of variable
length and all protrude downwards where they encounter the underlying epithelial
layer and act as mechanoreceptors, being responsible, apparently for sensing
the distance between two adjacent epithelial layers and accordingly regulating
the local air pressure. It seems that the variable length of the fringes enables
to differentially determine the distance from one epithelial layer to the next
and also gauges air pressure extent in the system.
References
-Croitoru N, Ishay JS, Arcan L and Perna B (1978). Electrical resistance
of the yellow stripsof social wasps under illumination. Photochem. and Photobio.
28 (2): 265-270.
-Duncan CD. (1939). A contribution to the Biology of North American Vespina
wasps. Stanford University Press. Stanford University, California.
-Edwards R. (1989). Social wasps. Rentokil Ltd. East Grinstead.
-Egli PH (1960). Thermoelectricity. US Naval Research Lab. Washington D. C.
John Wiley and Sons, New York. 858 pp.
-Farley RD, Case JF and Roeder KD (1967). Pacemaker for tracheal ventilation
in the cock roach, Periplaneta americana. J. Insect. Pysiol. 13: 1713-1728
-Goldstein O, Litinetski L and Ishay JS (1996). Extraretinal photoreception
in hornets. Phy siol. Chem. Phys. & Med. NMR 28: 129-136.
-Goldstein O and Ishay JS (1996). Morphology of a putative new peripheral photoreceptor
in social wasps. Physiol. Chem. Phys. & Med. NMR 28 (4): 255-266.
-Grass‚ PP (1976). Trait‚ de Zoologie. Mechanoreceptors and mechanoreception
8 (3) pp. 543-595. The respiratory Apparatus, 8 (4) pp 93-204 , Masson , Paris.
-Gutmann F and Lyons E (1981). Organic semiconductors. Part A. Robert E. Krieger
Publ. Comp, Malabar, Florida, John Wiley and Sons, New York,
-Heinrich B (1981) Insect thermoregulation. John Wiley and sons , New York
-Huber F. (1960). Experimentelle Untersuchungen zur nerv"sen Atmungsregulation
der Orthopteren (Saltatoria Gryllidea). Z. v. Physiol. 43: 359-391.
-Imms AD. (1960). Entomology. Methuen & Co Ltd., London .
-Ishay JS. (1964). Observations sur la biologie de la Guˆpe orientale Vespa
orientalis en Israel. Inesectes Sociaux XI: 193-206.
-Ishay JS, Bytinski-Saltz and Shulov A (1967). Contributions of the bionomics
of the Orien tal hornet Vespa orientalis. J. Entomol. II: 45-106.
-Ishay JS and Ruttner F. (1971). Die Thermoregulation im Hornisennst. Z. v.
Physiolog. 71: 423-434.
-Ishay JS. (1972). Thermoregulatory pheromones in wasps. Experientia 28 (10):
1185-1187.
-Ishay JS (1973). Thermoregulation by social wasps: Behaviour and pheromones.
Trans. New York Acad. Sci. 35 (6): 447-462.
-Ishay J and Croitoru N (1978). Photoelectric properties of the yellow strips
of social wasps. Experienti-a 37: 340-342.
-Ishay JS, Perna B, Hochberg Y and Goldstein (Assanta) (1980a). Photoelectric
properties of the yellow strips in Vespa orientalis; a mathematical model. Bull.
Math. Biol. 42 (5): 681-689.
-Ishay JS, Shimony TB, Shechter OS and Brown MB (1981). Effect of xanthine and
col chicine on the longelivity, photoconductive properties and yellow pigment
structure of the Oriental hornet (Vespa orientalis). Toxicol. 21: 129-140.
-Ishay JS, Shimony (Benshalom) T., Lereah Y. and Duby T. (1982). Temperature
depen dence of electrical resistance of hornet and ant in low temperature: Direct
cuticle measure ments. Physiol. Chem. Phys. & Med. NMR 14: 343-361.
-Ishay JS, Benshalom-Shimony T, Kristianpoller N and Weiss D (1988). Luminescence
of the Oriental hornet Vespa orientalis. J. Luminescence 40 & 41: 221-222.
-Ishay JS, Benshalom-Shimony T, Ben-Shalom A, Kristianpoller N ( 1992). Photovolatic
effects in the Oriental hornet. J. Insect. Physiol. 38 (1): 37-48.
-Ishay JS, Rosenzweig E and Fuksman E (1995). Thermo-and photoelectric current
in hornet cuticle. Physiol. Phys. Chem. & Med. NMR 27: 179-192.
-Ishay, J. S., Rosenzweig, E. and Solomon, A. 1997. Thermoregulation of the
extraretinal photoreceptor apparatus in the yellow stripes of the gaster of
hornets. Phys. Chem. Phys. Med. NMR., 29 (2): 213-230.
-Ishay JS and Ganor E. (1992). External micromorphology of the frons plate and
its adja cent areas of workers of the Oriental hornet. J. Morph. 212 (1): 1-13
.
-Ishay JS, Pertsis V and Lentov E. (1994). Duration of hornet sleep induced
by ether anes thesia curtailed by exposure to sun or UV-radiation. Experiencia
50 (8): 737-741.
-Ishay JS and Barenholz-Paniry V. (1995). Thermoelectric effects in hornet silk
and ther moregulation in hornet's nests. J. Insect. Physiol. 41 (9): 753-759.
-Ishay JS and Litineski L. (1996). Thermoelectric current in hornet cuticle:
Morphological and electrical changes induced by temperature and light. Physiol.
Chem. Phys. & Med. NMR 28: 55-67.
-Ishay JS, Goldstein O, Rosenzweig E, Kalicharan D and Jongebloed WL. (1997).
Hornet yellow cuticle microstructure: A photovoltaic system. Physiol. Chem.
Phys. & Med. NMR 29: 71-93.
-K"ninger N. (1977). Signals from brood in social Hymenoptera. Proc. VII th
Int. Congres I. U. S. S. I., Wageningen (The Netherlands), pp 280-282.
-Kristianpoller N, Goldstein O, Litinetski L and Ishay JS. (1995). Light curtails
sleep in anesthetized hornets: extraretinal light perception. Physiol. Chem.
Phys. & Med. NMR 27: 193-201.
-Krombain KV. (1967). Trap-nesting Wasps and Bees: Life histories, Nests and
Associates. Smithoni-an Press, Washington.
-Lebrun D. (1971). Glandes tergles et surfaces curticulaires correspondantes
chez la Ter mite a cou jaune. Calotermes flavicollis (Fabr.). C. R. Academ.
Sc. Paris, 272: 3162-3164.
-Matsuura M and Yamane S. (1990). Biology of the Vespine Wasps. Springer-Verlag,
Berlin.
-Miller PL. (1965). The central nervous controlof respatory movements. In: The
Physiol ogy of the insect central nervous system. (J. E. Treherne & JWL
Beament , eds.). Acad. Press, London ; pp 141-245.
-Moses RA. (1975). Adler's Physiology of the eye. C. V. Mosby Comp., New York.
6th ed.
-Rosenzweig E, Fuch C and Ihay JS (1985). Electrical resistance of hornet cuticle:
chan ges induced by xanthines-a statistical model. Physiol. Chem. Phys. and
Med. NMR 17: 435-449.
-Shimony TB and Ishay JS. (1981). Thermoelectric (Seebeck) effect on the cuticle
of social wasps. J. Theor. Biol. 92: 497-503.
-Shimony TB and Ishay JS (1984). Electrical capacitance in hornets integument:
frequency, light and temperature dependence, possible p-n junction effects.
Physiol. Chem. Phys. 16 (4): 333-349.
-Snodgrass RE (1925). Anatomy and Physiology of the honeybee. McGraw-Hill, New
York.
-Spradbery JP. (1973). Wasps. Sigwick and Jackson, London.
-Ugolini A and Cannici S. (1996). Homing in paper-wasps. In: Natural History
and Evolu tion of paper-wasps. (S. Turillazzi and M. J. West-Eberhard, eds),
Oxford, 7: 126-146.
-Vecht J. Van der. (1968). The terminal gastral sternite of female and worker
social wasps (hymenop-tera, Vespidea). Proc. Kon. Ned. Acad. Wetensch, Amsterdam
(The Nether lands), 71: 411-422.
-Wigglesworth VB. (1963). A further function of the air sacs in some insects.
Nature 198: 206.
-Wilson EO. (1971). The Insect Societies. Belknap, Harvard , Mass (USA)