The measurements were performed on the stripes of the abdominal cuticle of
the hornets on the dorsal side or on the frons or clypeus plates. The stripes
on the 4th and 5th abdominal segments partly contain pigment cells situated
above the basement membrane and filled with yellow pigment granules (Becker,
1937; Ziegler and Harmsen, 1969). In social hornets (Vespinae) there are stripes
with a colored pigment differing from the background coloration of the rest
of the cuticle which normally is brown or in shades between red and black (Fig.
1). The colored stripes contain pigment granules underneath the translucent
cuticle where light sensila were detected (Ishay et al., 1986). These granules
are cylindrical in shape and in V. orientalis they comprise of what seems
to be spores of a symbiotic fungus (Ishay and Shmuelson, 1994). In the hornet
the pigment is of a prominent yellow color but in other hornets or wasps the
pigment can appear in various shades of green, beige, black (Vecht, 1957, 1959;
Ishay et al., 1967; Kemper and D"hring, 1967; Wilson, 1971; Matsuura and Sakagami,
1973; Spradbery, 1973; Edwards, 1980; Akre et al, 1981; Brian, 1983; Matsuura
and Yamane, 1990).
After numerous cuticular properties were characterized, it was found that active
or narcotized, live as well as dead hornets, produce voltages of several hundred
mV, a current of up to several mA, and the appropriate power. The electric resistance
of the hornet cuticle suggests the properties of semiconductors where the electric
carriers are electrons or holes (Hannay, 1959; Cope, 1965; Kittel, 1968; Watson,
1969; Gutmann and Lyons, 1981; Gutman et al., 1983; Ishay et al., 1991), apart
from their being endowed with a very large electric capacitance relative to
their mass. Of hornet components examined, the cuticle (and also the silk produced
by the pupating larvae) has a very complex chemical and structural constituent
which hinders observations during its formation (Rudall, 1963; Locke, 1966;
Anderson, 1974, 1979, 1985; Neville, 1975; Filshie, 1982; Schaeffer et al.,
1987).
The studies mentioned and numerous others deal with the biochemistry of the
insect cuticle or with its formation. In the present study we concentrated on
the structure of regions in the abdominal cuticle on which we have previously
performed electric measurements and which we intend to provide a better understanding
for the observed structures and the functional role they may possibly fulfill.
We already know that the hornet cuticle and pupal silk pick up solar energy
and convert it into other forms, as into electric energy which is probably used,
interalia, for cooling or warming the nest or for other daily needs (Ishay and
Barenhoiz-Paniry, 1995
Materials and Methods
Transverse stripes of cuticle from the abdominal segments 4 and 5 at
their dorsal (yellow) side were taken from anesthetized or dead hornets, see
Figure 1. Strips detached from their cuticle were fixed in a 0.1M cacodylate
buffered glutaraldehyde solution (pH 7.4; 2 hrs) for light microscopical (LM)
observation (Diavar, Reichert, Austria). For conventional scanning electron
microscopical (SEM) observation, strips were postfixed in a 2% osmium tetroxide
solution in the same buffer for 4 hrs, dehydrated in ethanol, critical point
dried in liquid CO 2 and sputtercoated with 10- 15 nm Au/ Pd. Samples were investigated
in a JEOL SEM (type 35) or a Cambridge stereoscan (type 1805) operated at 15-25
kV .
For field emission scanned electron microscopy (FE-SEM), glutaraldehyde prefixed
strips were immersed in a mixture of arginine HCL, glycine, sucrose and sodium
glutamate (2% each) for 16 hrs at RT, followed by rinsing in distilled water
(3x). Subsequently strips were immersed in a mixture of tannic acid and guanidineHCL
(2% each) for 8 hrs at RT, rinsed in distilled water 3x) and immersed for 8
hrs at RT in a 2% OsO 4 solution in distilled water, as described previously
(Kalicharan et al., 1992; Jongebloed et al., 1996). Finally strips were dehydrated
in ethanol and critical point dried in liquid CO 2 and sputtercoated with only
23 nm Au/ Pd. Samples were investigated in a JEOL FE-SEM, type 6301F operated
at 2-3 kV.

FIGURE 1. V. orientalis, showing their abdominal segments. Part of segments
4 and 5 are usually of a yellow color. The hornet on the left with the extruded
stinger (a) has been damaged, probably during the collection from the field
and consequently the two yellow abdominal segments display black areas of hemorrhage,
whether on the left in segment 4 or on the right in segment 5. (x3)
Results
In Figure I is shown a macroscopic image of the abdominal segments 4
and 5 at their dorsal side in two hornets. Figure 2 presents a crosssection
of abdominal segment no. 4 from its dorsal aspect in SEM. The figure shows a
general view of the cuticle, with the epicuticle on top and beneath it the exocuticle
which is constructed as a trabecular layer and the endocuticle which is a lamellar
layer. Underneath the endocuticle are the sinuses comprising the cavernous layer
(c) and at the bottom are the hypocuticle (h) and the basement membrane (b).
Additionally, one can see that in the bottom part, within the cavity of the
sinuses. there are 'pillarettes', or actually extensions of the pore canals
(pc) which pass from the epicuticle to the hypocuticle. (The yellow pigment
granules were removed during the preparation for SEM viewing.) Figure 3 presents
a section through the cuticle of abdominal segment 4 (displaying a strip of
yellow pigment). In the epicuticle several depressions can be seen, which are
the pores (p). At the base of the lamellar endocuticle one can see the clump
of yellow granules deriving from the pigment cells that occupy the spaces within
the cavernous layer. In Figure 4 we see a cross section through the exocuticle
and more interiorly, also through the endocuticle. The two uppermost layers
in the section are the thickest. The endocuticle (the lamellar layer) is composed
of 30 or more lamellae which become attenuated (thinner), the deeper we proceed
(i. e., the closer to the abdominal cavity). Thus, the upper layers are about
5 Ám in thickness whereas the lower layers are only about 1/ 3 Ám thick. Generally,
the lamellae are arranged in parallel shapes with a clear demarcation between
two adjacent ones, however, at intervals of several Ám one discerns also trabeculae
which interlink two or more lamellae. Clear separation between the various layers
can be seen in Figures 11 through 18. We need to point out that 'our' hornets
were collected from a natural nest in the field by a method described earlier
(Ishay, 1964) and among them were specimens inadvertently damaged, their cuticle
showing a black spot indicative of oxidized hemolymph. In cases where the segment
is damaged, whether the yellow distal part selectively or both the yellow and
the brown parts (see Figure 1), then there is blackening of the entire segment,
which tests to the presence of hemolymph that has come in contact with oxygen
(as occurs during any external injury).
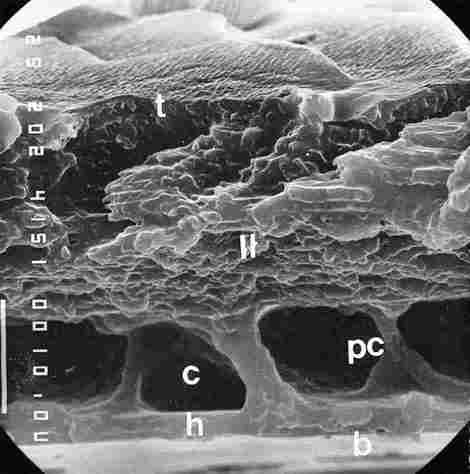
FIGURE 2. Cross section (or more precisely a transverse fracture) in a cuticular
yellow stripe. From top to bottom one can see epi + excocuticle (t), endocuticle
(II), then underneath a region of cavernae (c) and in between there are the
collumelles which are extensions of the pore canals (pc). At the bases of the
collumelles there are dilatations in their points of contact with the hypocuticle
(h). The lowermost layer is the basement membrane (b). Bar= l0 Ám.
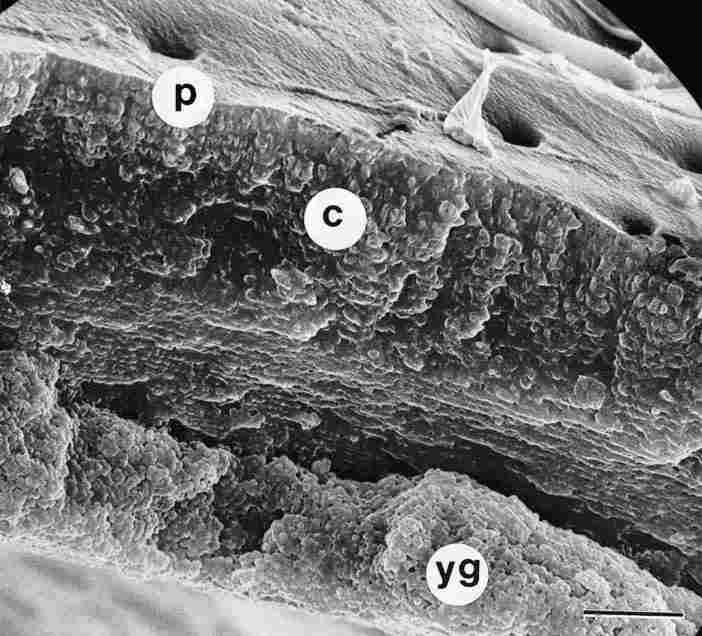
FIGURE 3. Cross section (fracture) through yellow cuticle. On top, in the epicuticle,
one sees a number of apertures, pores (p). On closer inspection one can detect
the layers of the endocuticle, which are laminar and separately beneath them
there is a mass of yellow pigment granules (yg) located within the sinus of
the cavernous layer. Underneath all this, one can vaguely make out the bottom
part of the cuticle. c = cuticular layers. Bar = 10 Ám
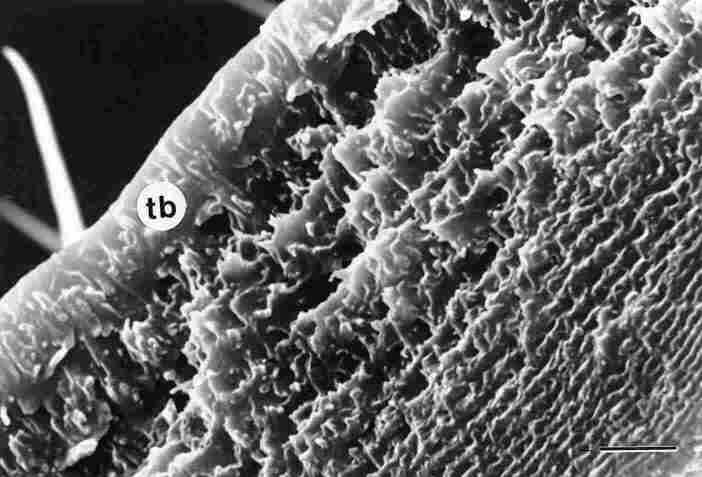
FIGURE 4. The previous figure, tilted upside down (180░) and at twice magnification.
Now one sees the exocuticle with the trabeculae (tb) arranged longitudinally.
This is the trabecular layer. This layer and the one immediately below it are
each about 5 mm in thickness. The further one proceeds downwards (i. e., the
closer to the inner side of the gaster) the thinner become the layers of the
endocuticle, which we arranged in laminar fashion. Bar = 5 Ám.
In Figure 5, the exterior of the cuticle, that is, the epicuticle is seen.
At intervals of 10-20 (or sometimes 10-100 or more) Ám one discerns pores (p)
which are shielded on the cephalic side by eaves (i. e., light can be transmitted
inside the pore from the upper or posterior side only). Between each two rows
of pores, we can see setae( s). The top layer of the epicuticle is arranged
in the form of shingles (or scales). Figure 6 presents a section through the
epi and exocuticle. The epicuticle (ep) is built as a continuous plate of about
1 Ám in thickness; it comprises the topmost layer of the cuticle which comes
in direct contact with the environment. Underneath this plate there is the exocuticle,
which is comprised of trabeculae that are arranged perpendicularly to the upper
plate. The trabecular layer is about 5 Ám thick. The outlet of the pore (p)
is located in this region. At the top, one can see a part of the canal that
surrounds the pore, the socalled pore canal (pc) and more in depth the hollow
area of the pore which here is less than 1 Ám in diameter. In the lower portion
of the cavernous layer the pores broaden out into a sort of pedicle as shown
in Figure 7. After removal of the hypocuticle from the bottom of the pore Figure
8, the various layers (c) comprising the pore canal are exposed. Here, too,
one discerns the sealed terminus of the pore canal (pc) with the gap remaining
to admit light (1). The maximal width in this region is about 20 Ám. At this
depth one can discern strands which interconnect the tips (lower side) of the
various pores. These interlinking strands apparently are tracheae (tr), Figure
9. Closer to the exterior of the inner surface of the cuticle (i. e., to the
abdominal cavity) one can see, under proper illumination, a reflection of the
light shining through the cuticle on the other side. Between the lightadmitting
gaps around the pores there are delicate fibers, apparently the fibers of neurons
and bipolar cells (bp),
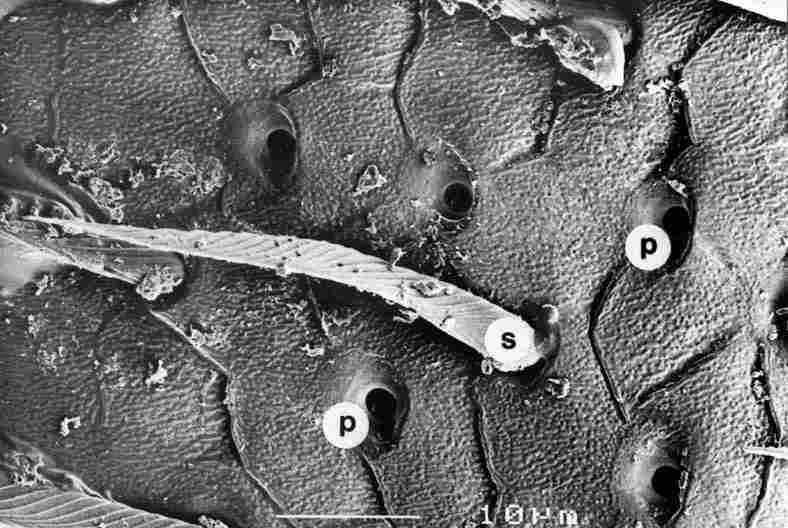
FIGURE 5. Figure showing apertures of the pores (p) from the top. Diameter of
the pore aperture is about 1-2 mm and they me dispersed at intervals of 10-50
mm or more. The pore is actually located within a depression and usually possesses
a cave in the cephalic direction (which here is to the right), Between the rows
of pores are interspersed setae (s). Bar = 10 Ám.
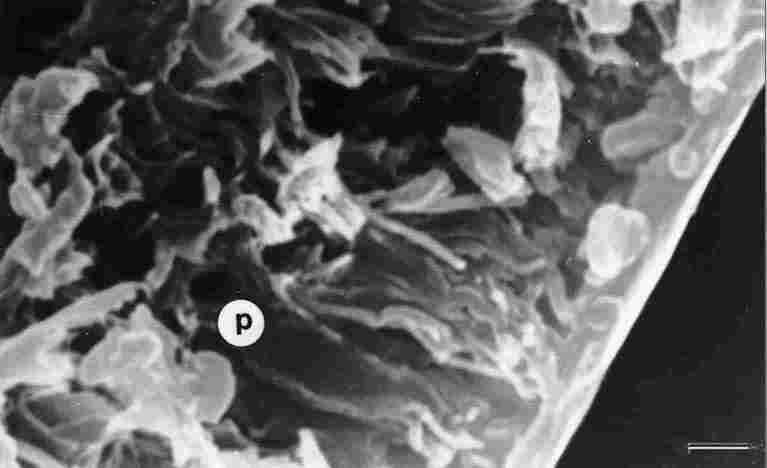
FIGURE 6. Section (fracture) through the executicle (or trabecular layer). Between
the various rifts that pass here from below, first is seen a part of the pore
(p). Then one can see the pore canal (pc), which is rounded and extends to a
depth of 4-5 Ám. ep = epicuticle. Bar = 1 Ám.
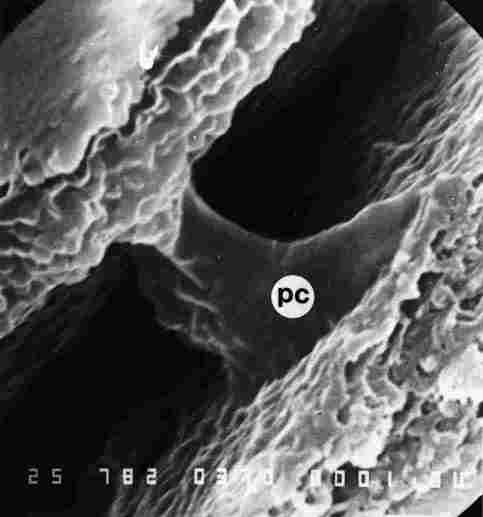
FIGURE 7. The pore canal (pc) in the region of the cavernous layer. From the
top one sees the bottom layers of the endocuticle. Note that in the lower part
of the endocuticle the canal commences at top at a diameter of 1.5-2.0 mm but
becomes much wider further down. This is the region in which the photoreceptor
cells are encountered.
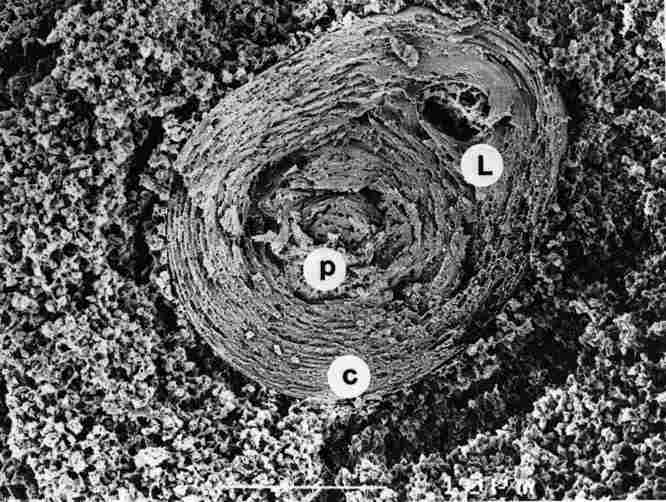
FIGURE 8. Cross section (fracture) through the lower part of the pore canal
(pc), in the region where the central concentric layers (c) me already closed
but in a brief eccentric region (off-center, to the right) there is still an
opening (L). Around the pore canal there are granules of yellow pigment (yg).
Bar =1 Ám.
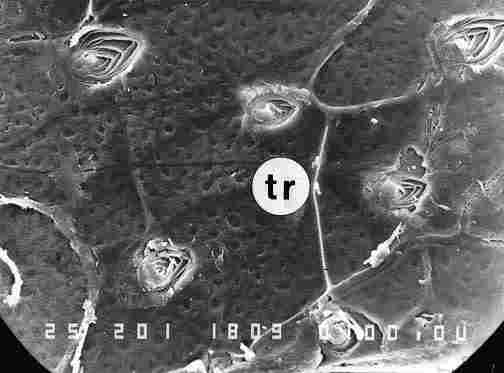
FiGURE 9. After scraping off the layers of the hypocuticle at a slightly lower
level than that shown Figure 8, we can see the still not closed layers of the
pore canal, probably the tracheae (tr). Bar = 10 Ám.
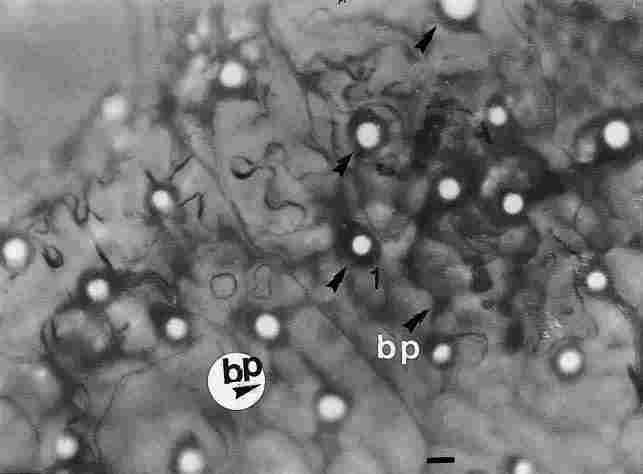
FIGURE 10. An image of the reflection of light passing through the pore canals
on the bottom side the cuticle. The picture was taken through a light microscope
in the region above the basement membrane. The picture shows clear circles (see
arrow) which are the emergent light beams. Around them there are dark circles
(see arrows) representing a ring of tracheae and nerve fibers as well as nerve
fibers that reflect through the basement membrane and include also bipolar (bp)
cells. Bar =10 Ám
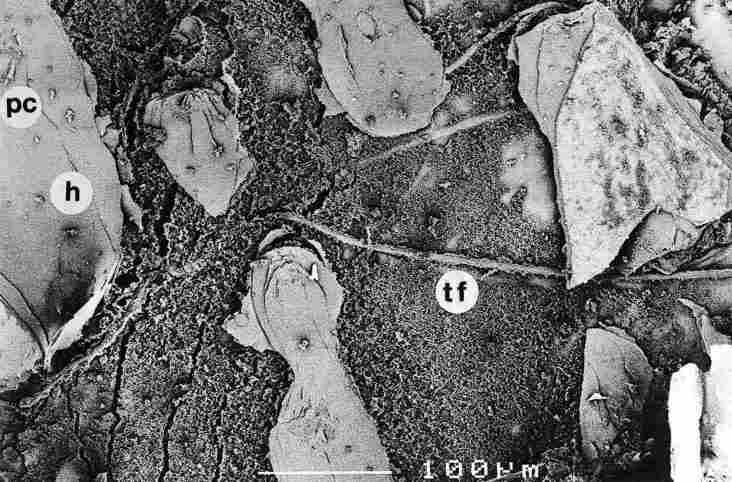
FIGURE 11. After removing a number of layers of the hypocuticle, one sees the
boutons of the scaled-off photore-ceptor (arrow), pc-pore canal; h-hypocuticle;
tf-tracheal tube. Bar = 100 Ám.
Figure 10. Thus, these neurallike fibers are located between the basal membrane
and the hypocuticle, whereas the tracheae are situated here, also more deeply,
between the hypocuticle and the cavernous layer. A broader view of the basis
of the cuticle in these regions is given in Figures 11-16. In Figure 11, where
the basement membrane and several layers of the hypocuticle had been removed,
we can see thick fibers that penetrate between the layers, the tracheal tubes
(tt). We can also see the termini (sealed) of the pore canals (pc) interspersed
between the thin layers of the hypocuticle. In Figure 12, we see at higher magnification
at 'a', very thin layers of removed hypocuticle (h) and underneath them rounded,
papilliform bodies, which are the termini of the pore canals (pc), and around
a 'sea' of yellow granules (yg). We can also observe traces of tracheae (tt).
In Figure 13 details of a completely sealed single pore canal (pc) can be seen.
This sealing is only gradually can be judged from the thin concentric layers
at the distal end of the pore canal (a) and above them the internal layers (b)
which are higher up and responsible for the sealing, with a single strand (s).
The lower, concentric layers gradually merge into the plates of the hypocuticle
(h). The image shows five of these leaflike layers around the pore canal. One
can also see the yellow pigment granules (yg). When we remove the 'lid' of the
pore canal (pc) in its eccentric region we notice, that it is surrounded by
granules of yellow pigment (yg) and that the various layers composing the pore
canal are rather sparse and gapped, leaving room for the passage of light (see
Figures 8, 9, 10) between the hub of the pore canal and layer 1 and between
layer 1 and layer 2 (from the center). The cuticular layers here are rather
thin and the exterior most conjoin to form horizontal plates where previously
they were arranged vertically. Figure 15 presents a detail of the cuticular
plates, taken from the eccentric region which trans mits light. We can see lacunae
of several sizes in the center of the pore canal (pc) and also between the more
distal plates (a, b, c). In each cuticular plate one discerns a central, porous,
core which is flanked by two walls in sandwich fashion (s). Each plate shows
both transverse (tr) and longitudinal (lo) hole which apparently are conduits
for hemolymph. In Figure 16, we see at higher magnification a layer of plates
in the dense region from which it is obvious that in cross section each cuticular
plate is comprised of two very thin walls. Between the walls the porous plate,
takes up most of the space. The thickness of each plate here is about 1/ 3 Ám
and its content is porous with numerous apertures, which seem to be necessary
for the transport of hemolymph. An enlargement of what was shown in Figure 13
is given in Figure 17. At the upper margin and left side of the figure granules
of yellow pigment (yg) can be seen, while at the center we can see in more detail
the closure of the pore canal. At the margins the concentric plates of the canal
are shown, which abruptly change their orientation from vertical to horizontal.
Note, that only the thin plates undergo this change in orientation, conjoining
to form the hypocuticle. Here, too, one observes apertures traversing the plates
(arrows). Figure 18 provides a higher magnification of Figure 16. On the top
right we see yellow pigment granules and to the left of these are visible part
of the concentric plates which surround the pore. In some of these plates one
can distinctly observe longitudinal apertures (1a). We also note that the pore
canal separates between the mass of yellow granules (yg) and the cavity of the
pore.
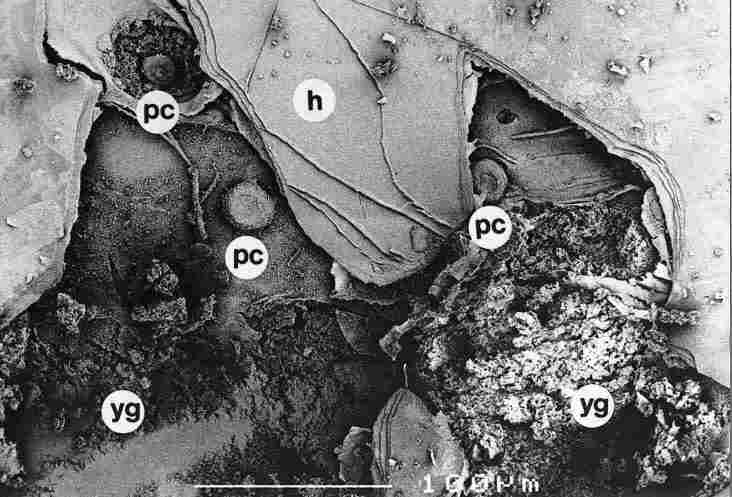
FIGURE 12. One can discern the closure boutons (arrow), with one of them (a)
surrounded by granules of yellow pigment (yg). This "bouton" remained intact
(a); besides a broad part representing the closure area it possesses also a
short and narrow papilliform extrusion (arrow) of avery small diameter. "is
latter is the contact point through which apparently drains the electric energy
stored in the capacitor in the upper cuticle. For greater detail consult text.
pc-pore canal, tt-tracheal tube, h-hypocuticle. Bar = 100 Ám.
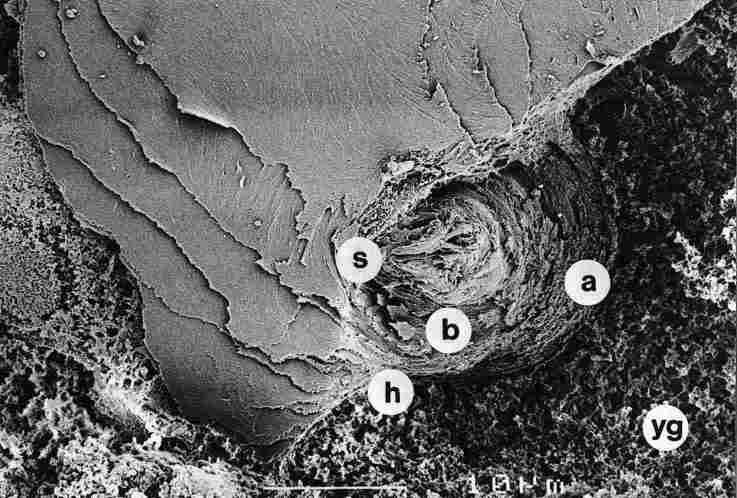
FIGURE 13. At higher magnification than at Figure 12 we see a "bouton" whose
central part is sealed. Here one finds the thick layers of the hub of the pore
canal, whereas the thinner layers (a) stemming from the interior of the cuticle
(the lamellar layer) recurve to "open" anew after the closure so as to form
the hypocuticle (h). Around the pore canal (pc) and below the hypocuticle, one
sees an abundance of yellow granules (yg). b-internal layers of the pore canal.
Bar = 10 Ám.
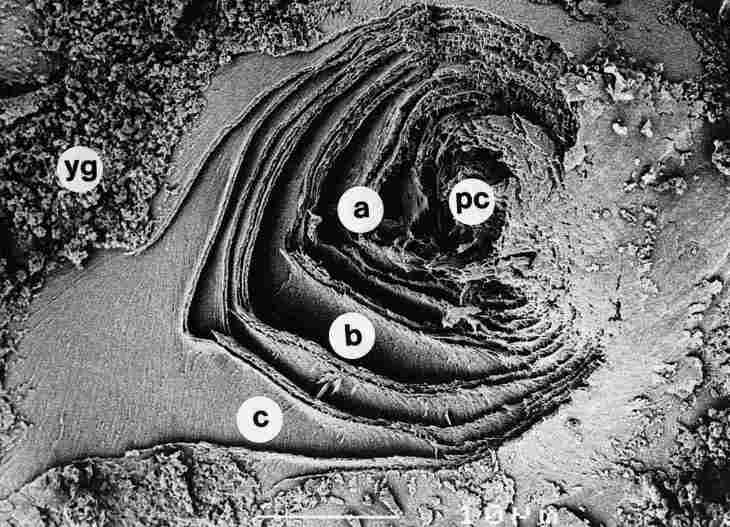
FIGURE 14. A view from a plane lower than that of a pore canal (pc) closure.
One notes that the cuticle layers (a, b and c) are concentric and gapped, with
yellow granules (yg) around them. Bar =10 Ám
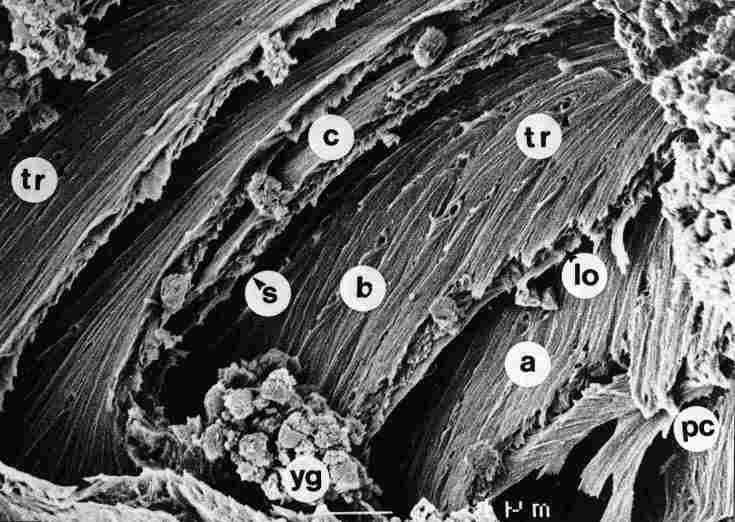
FIGURE 15. A view of the thinner layers around the pore canal (pc) prior to
the closure. In each cuticular layer a, b or c, whose thickness is less than
1/ 3 mm, there are two smooth walls and an apparently porous center. At various
intervals (of 1 mm or more), we see apertures (tr) that traverse the cuticle
and around them yellow granules (yg). lo-longitudinal pore, s-free space between
two lamellae. Bar = 1 Ám.
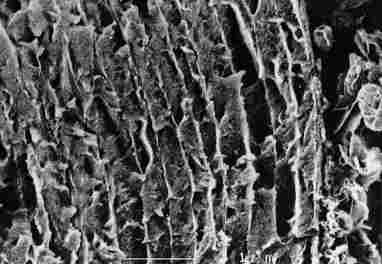
FIGURE 16. Cross section of the pore canal wall in the closure region, The thickness
of the layers here is about 1/ 3 mm, and each layer displays two walls and a
content-filled space in between. In some layers the inner part shows apertures
(*) which probably serve for the passage of hemolymph. Bar = 1 Ám.

FIGURE 17. The region of a pore canal closure (pc) is shown and surrounded by
thinner layers, which at the closure point 'reopen' to create the thin plates
of the hypocuticle. Here, too, can be seen delicate apertures (tr) which traverse
the width of the layers. All around one sees yellow granules (yg). Bar =1 Ám.
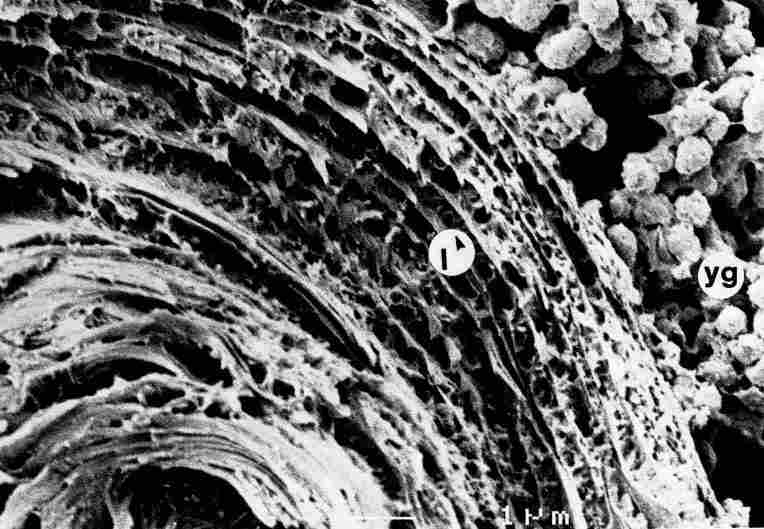
FIGURE 18. In some layers of the pore canal one gets a clear impression of the
apertures which traverse the length of the cuticle (arrow). Bar = 1 Ám.
If we photograph the terminus of the pore after its closure point, that is,
underneath the hypocuticle, we can observe that this pore canal termination
becomes tapered toward the tip (as observed in Figure 19). The entire structure
(i. e., the pore) is separated from the environment by a ring (b), yet is linked
to the environment by delicate fibers (1, 2, 3). The tip of the pore canal (d)
appears to have a diameter of 2-3 Ám, which is about the width of the pore canal
at its commencement in the exo and endocuticle. Viewed from below, the entire
structure is seen to be supported by various cords (c) arising from the inner
borders of the pore canal. When we evacuate (by vacuum) the content of the pore
canal, we can see its encasing walls (Figure 20).
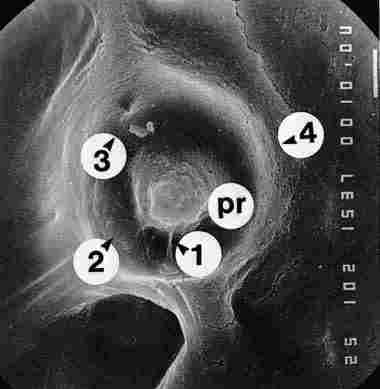
FIGURE 19. Preparation of a cuticle with yellow pigment from which the lower
portion the hypocuticle had been removed. There is a clump of yellow granules
(yg) at the basal surface of which one sees a crater which once housed the pore
canals of. The crater is broad at this basal site and must have contained the
photoreceptor cells. The entire structure is supported by cords (c) (1, 2, 3).
Bar = 10 Ám.
Figure 21 is a drawing of what the LM-SEM images have revealed. The pore canal,
inner structure of the tip of the pore canal, the pigment granules and the various
leaflike layers encasing the pore canal are shown.
Discussion
In the present paper, we have provided a detailed description of the
structure of the hornet cuticle in the region of the yellow stripes, that are
known, as a semiconductorlike material (Ishay and Croitoru, 1978). The upper
portion of the epicuticle is flat and continuous, barring the region of the
pores. As for the exocuticle, it has vertical structures, namely, trabeculae,
which provide mechanical support. There are 30 or more parallel layers rolled
around the abdomen, whose general shape from below resembles a cone. These layers
which are transparent or translucent extend down to the region of the yellow
pigment granules. The upper part of the abdomen is convex, producing a lenticular
shape that focuses the iff achated light on the inner, yellow pigment granules,
i. e., similar to a 'Fresnel lens' (Maycock and Stirewalt, 1981). The cuticle
is photovoltaic (Ishay et al., 1992), the voltage accumulates in the lower parallel
lamellae whence it is transmitted to the walls of the pore canals. These walls
descend to below the yellow pigment layer whose granules absorb all visible
light except the wavelength of yellow (that is reflected) so that, most probably,
underneath them there is darkness. The thicker (upper) layers of the cuticle
close off in the bottom part of the pore canal, while the thinner layers beyond
the closure point reopen. At an angle of 90 0 C forming thin plates of the hypocuticle,
sealing off the 'sandwich' from below. This photovoltaic system is active in
daytime for most of the hornets lifetime, i. e., for workers several months
during the warm seasons while for the queens a whole year. The 'sandwich' proper
is comprised of about 30 (or more) horizontal layers that are doped with Si,
P, S, Cl, K and Ca and smaller amounts of Mg, Fe and Zn, some of them are electron
donors, others electron acceptors. The parallel layers progressively attenuate
from the exterior down to the yellow pigment layer, which is likewise horizontal.
In the vicinity of the pore canal, however, all layers become vertical and contribute
to the formation of the walls of the pore apparatus. The multilayer walls are
built as biological mirrors, i. e., they reflect the incident light due to their
optical thickness of about onequarter of the wavelength of light (Land, 1972,
1981). This mechanism protects the content, i. e., the photoreceptor from overheating,
and so also the whole insect body. Regarding the question as to which light
wavelengths are reflected by the cuticle, we need to note that the various cuticular
layers range between 5 Ám and 0.3 Ám and possibly even down to 0.2 Ám, in thickness
(see Figures 4, 16, 17, 18). As is known, infrared light is emitted by radiation
from a heated surface. The region from 0.75 Ám to 1.2 Ám is called the photographic
infrared, because photographic emulsions still respond to radiation of such
wavelengths. Arbitrarily, it is customary to divide infrared light beyond the
photographic infrared into 3 ranges, namely, near infrared, at a wavelength
range of 1.2-.2 Ám and beyond that, the far infrared, which ranges between 8-14
Ám ( between 5.2-8 Ám the atmosphere is entirely opaque). On the assumption
that thickness of the cuticular layers represents a quarter of the wavelength
which it reflects, then the layers closest to (i. e., the innermost layers)
reflect the near infrared (the photographic infrared) because 0.2 Ám x 4 = 0.8
Ám or 0.3 Ám x 4 = 1.2 Ám, but the outermost layers which are 5 Ám thick are
accordingly apt to reflect the waves of the far infrared (5 Ám x 4 = 20 Ám).
It follows that the hornet cuticle utilizes at least part of the visible spectrum
as (electric) energy source, whereas the infrared waves which are a heat source
are reflected, which enables the hornets to fly undisturbed in the daytime heat.
Detection of infrared radiation, whether of terrestrial or remote stellar origin,
is achieved in human technology either by relying on the energy state in semiconductors
where a photon lifts an electron to a conductive state and the detection here
is of the photon induced change of the conductivity of the detector type, or
by relying on a semiconductor which contains a p-n junction, where an electronhole
pair is formed in the vicinity of the junction (Kruse et al., 1963). The strong
field on both sides of the junction separates between the two carriers so that
a photovoltage is created. Such detectors are instigated on the photovoltaic
effect (Hudson and Hudson, 1975) in the cuticle of hornets which is itself photovoltaic
(Ishay et al., 1992) and also behaves as a semiconductor (Ishay et al., 1991).
Thus the hornet cuticle possesses, theoretically, the ability to detect infrared
radiation, whether it is for the purposes of gauging the temperature, or for
recognizing nest mates in the darkness of the nest, or for the purposes of communication
and navigation.
The pore apparatus includes the pore canal, whose hollow portion serves as
a light guide for the photoreceptor (Goldstein and Ishay, 1996) while its walls
conduct the electric energy formed in the illuminated portion to the bottom,
darkened part of the photoreceptor (the bulbous head of the arrow). The latter
attenuates into nippleshape in the region of the darkened hypocuticle. Here
the electrical energy is transformed either into a current transmitted to the
hypocuticle plates or into a combined voltage which is transmitted, interalia,
to the nerves that support the pore. In the dark the electric resistance, which
in light was at a level of giga ohms (GW) drops down to a level of kilo ohms
(KW) a decrease of about 56 orders of magnitude (Ishay and Litinetsky, 1996).
This difference prevents electrical current from flowing back into the photovoltaic
cells, i. e., in this respect behaving like a diode (BenShalom and Ishay, 1989).
The dielectric fluid permeating all the internal spaces is the hornet's hemolymph
which is transparent and of a yellow coloration (like that of the yellow granules).
The hemolymph of V. orientalis adults has a pH lower than 7.0, i. e.,
is acidic; the osmolality range is between 321-593 mOsmole/ kg and the specific
gravity is 1.022-1.028 (Joshua et at., 1973). However, in cases of damage to
the cuticle, the hemolymph darkens oxidizes, thereby preventing the transmission
of light (Whitcomb et at., 1974).
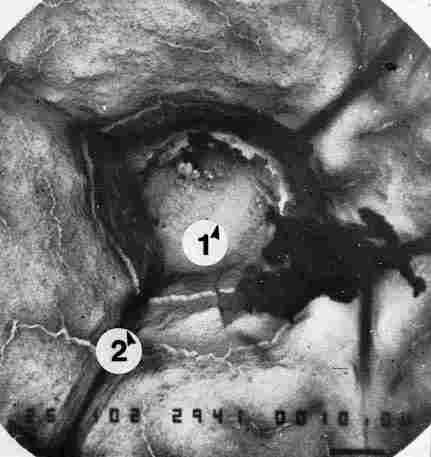
FIGURE 20. If one applies suction by vacuum to the region housing the photoreceptor
cell, it is possible that the entire cell could be sucked out, leaving behind
only the framework of cuticular layers making up the pore canal, in which the
photoreceptor was originally enclosed. 1 = site of the photoreceptor; 2 = nerves
and/ or tracheae Bar = 10 Ám.
As described, the cuticle of the Oriental hornet is constructed in the manner
of a photovoltaic cell incorporating or linked to an electric capacitor. It
seems to us, that each hornet (and in this respect probably each bee or ant),
as it departs the nest under insolation (i. e., sunlight irradiation), charges
its capacitor (in fact, its numerous capacitors) and before attaining the maximal
(breakout) voltage the insect must fly back to its nest and discharge some (or
most) of the energy it has accumulated. This discharge includes also: O 2 released
from the traps (see further) and, of course, the prey collected during the flight.
The discharge is probably achieved through contact of its feet with the silk
caps of the pupae which serve as a capacitor for the entire nest (Ishay and
Barenholz-Paniry, 1995), or by probing adult homers, at the entry or inside
the nest, with their antennae. As for the charge, this apparently is dependent
on various factors such as humidity, temperature, light irradiation, age of
the hornet and the like (Shimony and Ishay, 1984), and consequently the hornets
outside the nest apparently must return to the nest at time intervals, correlated
to the solar UV and blue radiation dose, which is different at different hours
of the day even at the same location. It seems to us (Ishay et al., 1967), that
during its lifespan each hornet is undergoing at least 3000-4000 cycles of charging
and discharging and in this respect it resembles a good battery.
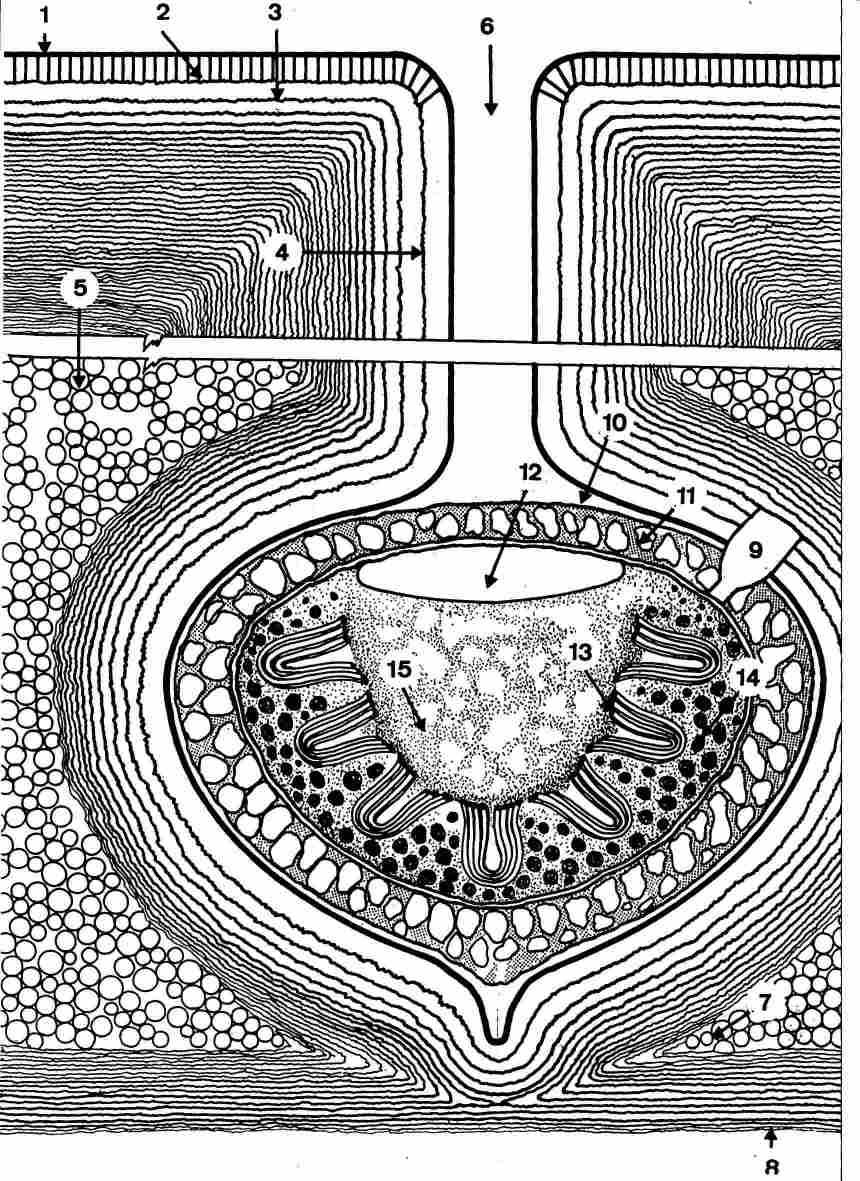
FIGURE 21. Schematic drawing of a section in the cuticle in the region of the
pore canal. 1-the epicuticle; 2-the exocuticle; 3-the endocuticle; 4-the endocuticular
layers which descend vertically into the pore canal; 5-the yellow granules layer;
6-the pore canal with a separating ridge running down its length which is about
30 mm; 7-the junction, i. e. the order line between the contact point (the end
point of the photoreceptor) and the hypocuticle and between them the gap is
filled with the yellow granules; 8-plates of the hypocuticle; 9-the synapse
in contact with the photoreceptor; 10-the gap between the photoreceptor and
the surrounding cuticular sinus; 11-the surrounding belt of yellow pigment around
the outer side of the photoreceptor cell; 12-the central area of the photoreceptor;
13-microlamellae; 14-dark pigment granule; 15-granulation at the center of the
photoreceptor.
The stripes of yellow cuticle are built and function as a photovoltaic system
(Ishay et al, 1992). The upper portion contains parallel transparent plates
not unlike the photovoltaic plates in known solar cells (Maycock and Stirewalt,
1981), except that in hornets it is composed of 30 or more photovoltaic plates,
which markedly enhances the efficiency of the system. The plates are thicker
in the upper part of the cuticle, therefore more electric carriers accumulate
in the thinner, lower plates. In this connection, thickness of the plates acts
much the same as the gap between two consecutive plates, that means, the greater
the gap or distance the smaller the capacitance. The electric charges in this
case probably electrons settle on both sides of each plate because of the energy
of photons which has boosted them from the valence band to the conduction band
of the yellow granules layer (= the anode) and in light they get caught in some
type of traps (perhaps O 2 ) that are formed in the cuticular plates (= the
cathode). For further details on traps consult Gutmann and Lyons (1981) or Gutmann
et al. (1983). The relative quantum efficiency of the lightinduced voltage as
a function of wavelength is highest at or around UV and blue light: the cuticle
is luminescent and when irradiated by UV light at 290 nm the emission spectrum
is maximal at 345, 450 and 510 nm and the D energy between the irradiated UV
and emitted light is absorbed and converted to other forms of energy by the
cuticle (Croitoru et al., 1978; Ishay et al., 1987; Ishay et al., 1992). At
high noon, when the relative amount of UV light in the radiation impinging upon
the earth is maximal, hornet activity is also at a maximum (Ishay and Lior,
1990). Additionally, we found that the short wavelengths (UV and blue) are the
ones that exert the greatest awakening effect on homers that had been anesthetized
by ether (Ishay et al., 1994; Ishay and Levtov, 1994; Kristianpoller et al.,
1995; Goldstein et al., 1996).
In the sixties it was noted by one of us (J. S. Ishay unpublished observations)
in a honeybee artificial flight room in the TelAviv University and subsequently
confirmed in the flightroont of the Institute für Bienenkunde, Oberursel/ Taunus,
Frankfurt, Germany, that hornets are attracted to UV light and show a high tendency
to fly or walk toward a UV light source even at daytime. Several electric UV
grid devices are now commercially available for insect control (see Edwards,
1980). UV lamps are strongly attractive to wasps as well. The sunlight is radiated
perpendicular to the cuticle at least in the upper part of the body. It traverses
all the layers of the upper parts of the cuticle; in the region of the yellow
stripes the cuticle itself is translucent and therefore the passing light is
absorbed in the layer of the yellow granules. The thickness of this layer is
about 5 Ám and there the photons 'push' out electrons and set them free. The
excess electrons move up and accumulate on the plates of the cuticle's horizontal
layers that become n-layers, because electrons have a negative charge. In the
yellow pigment layer therefore, holes are created, it becoming the p-layer.
As long as radiation falls upon the junction, electronhole pairs will be formed
and separated by the internal electrical field at the junction, i. e., it may
act as an infrared detector (Kruse et al.. 1963). One of the p-n junctions is
at the upper border between the yellow granules layer and the lower endocuticular
layer, i. e., a broad area junction. Most of these layers in the lower endocuticle
are about 0.3 Ám thick and the whole endocuticle is about 25-30 Ám thick, i.
e., optimal for a (commercial) solar cell (Maycock and Stirewalt, 1981). The
length of each yellow granule is about 0.5 Ám (Ishay and Shmuelson, 1994), i.
e., of optimum dimensions for absorbing photons in amorphous silicon. Beyond
the layer of yellow crystals light does not pass, so that the hypocuticle is
in darkness (apart from the pores which do transmit light). Thus, charging of
the cuticle is effected, with -at the exterior and + inside. By the mode of
hookup described for the various measurements (Ben Shalom and Ishay, 1989),
in more than 85% of the measured specimens, a positive voltage on the inner
(i. e. lower) surface of the cuticle compared to its outer surface was found.
The range of the obtained voltage was up to 0.4 V at open contacts, i. e., close
to that of a usual photovoltaic cell and a short current range of 0.1-5 mA.
The electric capacitance of the cuticle at 1000 Hz was about 0.6 nF as opposed
to 3.0 nF at 100 Hz. This inverse relationship between frequency and the electric
capacitance might point to the presence of polar substances possibly chargeable
proteins or ion pumps. The findings suggest that the yellow stripes in the cuticle
act as semiconductors of p-type while the brown stripes act like those of n-type
(Shimony Benshalom and Ishay, 1984). The current was also measured (in dead
specimens) in correlation with time in order to compute the electric charge
in the hornet (Ishay et al., 1990). It was found that even after 24 hours of
maximal current drain, the hornet is still not absolutely discharged. The drained
current yielded an exponential curve that enabled computation of the electric
capacitance app. 3nF, the chargeapp. 2.5 mCoulomb (mC) and the energyapp. 4.6
mjoule (mj). These measurements were performed at room temperature (i. e., 20-24
0 C) and at low relative humidity. Same measurements performed at the proper
optimal conditions yielded results that were higher by 2-4 orders of magnitude
(Ishay and Litinetsky, 1996). There is yet no information about these parameters
in living specimens measured in optimal conditions. This 'battery' which charges
itself with light energy becomes charged, of course, only when the hornets fly
in (or are exposed to) the sun, and the closer the flights to noon time the
faster the charging process, which means that the flying time is accordingly
curtailed (because the hornets must return to the nest to get fid of the excess
voltage before breakdown occurs in the 'battery'). As known, the cuticle behaves
as an intrinsic semiconductor and therefore it may he charged also in darkness
at the optimal temperature where the activating energy is (Ea) = 0.536-1.859
eV. In the hornet yellow cuticle there are in fact two systems of charging,
namely, that of the upper cuticle where the resultant electric charge leaves
through the layers of the pore canal to reach the hypocuticle, and that of the
yellow pigment granules which 'encounters' the cuticular layers in the dark,
lower region of the pore canal and conjoins with them to form a p-n junction.
Another p-n junction is at every contact of yellowbrown stripe of the cuticle.
It is tempting to locate the main junction at the 'contact point', the nipples
on the boutons. The current or voltage which flows from this 'solar battery'
can serve, while discharging, various roles, not all of which are presently
known to us. Yet it stands to reason that electric energy is used via a Seebeck
effect or other thermoelectric effects like Peltier and Thompson (already reported
in hornets) (see Shimony and Ishay, 1981a). Seebeck found (in 1821) that a magnetic
compass needle held close to a circuit made of two different conductors was
deflected thus indicating a flow of electric current when the two junctions
in the circuit were held at different temperatures. Peltier discovered, in 1834,
that when an electric current is passed through a junction between two electric
conductors, heat is either absorbed or is emitted at the junction, depending
on the direction of the electric current flow. Thompson, in 1857, discovered
a third thermoelectric effect related to the already mentioned two: the absorption
or evolution of heat when an electric current flows in a uniform conductor along
which there is a temperature gradient. The created electric energy heats or
cools the air passing through vespan tracheal tubes, which are abundant between
the plates of the hypocuticle (See Figures 9-12). Indeed in the hornet V.
crabro, one can actually see a tracheal fing encircling each photoreceptor
in its lower part (Shimony and Ishay; 1981 b), possibly contributing to maintenance
of an optimal temperature around each pore which contains an extraretinal photoreceptor
(Goldstein and Ishay, 1966) and thereby also contributing to self thermoregulation
of each hornet during its flight. Even when the hornet flies in the hot sun
in the hottest regions of the globe and at high noon (the period of main activity).
The only time that hornets are seen to imbibe water is when building their combs
(Ishay, unpublished). Yet the same mechanism can be activated, when necessary,
to warm the brood (primarily the pupae) by blowing warm air from the spiracles
(= the tracheal outlets) upon them (Ishay and Rutmer, 1971). In connection with
this intriguing prospect, we note that yellow stripes occur on all the species
of social hornets and wasps, and in many species of solitary wasps, not to mention
numerous other nonsocial insects of various taxa. Apparently the number of yellow
stripes in the cuticle of social or solitary insects is low in tropic and subtropic
regions however, increases with increase in the geographic latitude or increment
in the altitude. Furthermore, yellow stripes are more abundant in males than
in females and on the dorsum of the insects than on their ventrum and occur
particularly on the abdominal segments. We assume that there, they serve the
same purpose as conjectured above.
References
-Akre, R. D., Greene, A., MacDonald, J. F., Landolt, P. J. and Davis,
H. G. (198 1) The Yellow jackets of America North of Mexico. U. S. D. A. Agriculture
Handbook No. 552.
-Andersen, S. (1974) Cuticular sclerotization of larval and adult locusts, Schistocerca
gregaria. J. Insect. Physiol. 20: 1537-1552.
-Andersen, S. (1979) Biochemistry of insect cuticle. Anna. Rev. Entomol. 24:
29-61.
-Andersen, S. ( 1985) Sclerotization and tanning of the cuticle. In: Comprehensive
Insect Physiology, Biochemistry and Pharmacology, (G. Kerkut and L. Gilbert,
eds), Pergamon, Oxford, pp. 59-74.
-Becker, E. (1937) Uber das Pteridinpigment bei Insekten und die Farbung und
Zeichn vonVespa im besonderen. Z. Morph. Okol. Tiere 32: 672 -751.
-BenShalom, A., Eshed, C., BenshalomShimony, T. and Ishay, J. S. (1988) A theoretical
model of electrical properties of the Oriental homet cuticle. Physiol. Chem.
Phys. & Med. NMR 20( 3): 227-239.
-BenShalom, A. and Ishay, J. S. (1989) The homet cuticle as a diode and an electric
source Physiol. Chem. Phys. & Med. NMR 21: 95-106.
-BenShalomShimony, T. and Ishay, J. S. (1990) Luminescence and thermoconductive
properties of the Oriental homer cocoon: evidence for phominducedelectrontransfer
system. Comp. Biochem. & Physiol. 95A( 3): 349-358.
-Brian, M. V. (1983) Social Insects. Chapman and Hall, London.
-Cope, F. W. (1975) A review of the application of solid state physics concepts
in biologcal systems. J. Biol. Phys. 3: 1-41.
-Croitoru, N., Ishay, J., Arcan, L. and Pena, B. (1978) Electrical resistance
of the yellow strips of social wasps under illumination. Photochem. and Photobiol.
28( 2): 265-270.
-Edwards, R. (1980) Social Wasps. Rentokil Limited, Felcourt, West Sussex.
-Filshie, B. K. Fine structure of the cuticle in insects and other arthropods.
In: Insect Utrastructure clure, (R. C. King and H. Akai, eds), Vol. 1, Plenum,
New York, pp. 281-312.
-Fleissner, G. and Frisch, B. (1993) A new type of putative nonvisual photoreceptor
in the optic lobe of beetles. Cell and Tissue Res. 273: 435-445.
-Goldstein, 0. and Ishay, J. S. (1996) Morphology of a putative new peripheral
protorecetor in social wasps. Phlysiol. Chem. Phys. & Med NMR 28: 255-266.
-Goldstein, 0., Litinetsky, L. and Ishay, J. S. (1996) Extraretinal photoreception
in hornets. Physiol. Chem. Phys. & Med. AMR 28( 2): 129-136.
-Gutmann, F. and Lyons, L. E. (1981) Organic Semiconductors. Part A. Krieger,
Malabar, Florida.
-Gutmann, F., Keyzer, H. and Lyons, L. E. (1983) Organic Semiconductors. Part
B. Krieger, Malabar, Florida.
-Hannay, N. B. (ed) (1959) Semiconductors. Reinhold Publishing Corp., New York.
-Hudson, R. D. Jr. and Hudson, JW. (1975) Infrared Detectors. Dowden Hutchinson
and
-Ross, Inc., Halsted Press, a Division of John Wiley & Sons, Inc.
-Ishay, J. S. (1964) Observations sur Is biologic de la Guepe orientate Vespa
orientalis in
-Israel. Insectes Sociaux XI( 3): 193-206.
-Ishay, J. S., BytinskiSaltz, H. and Shulov, A. (1967) Contributions to the
bionomics of the Oriental hornet Vespa orientalis. Israel J. Entomol. 11: 45-106.
-Ishay, J. S. and Rutmer, F. (1971) Die thermoregulation im Hornisennest. Zv
Physiol. 72: 423-434.
-Ishay, J. S. and Croitoru, N. (1978) Photoelectric properties of the "yellow
strips" of social wasps. Experientia 34( 3): 340-342.
-Ishay, J. S.. Perna, B., Hochberg, Y. and Goldstein, M. (Asanta) (1979) The
effect of hornet venom on the photoelectric properties of hornet cuticle. Toxicon
17( 4): 407-411.
-Ishay, J. S., Perna, B., Hochberg, Y. and Goldstein, M. (Asanta). (1980) Photoelectric
prop erties of the yellow strips in Vespa orientalis: A mathematical model.
Bull. Math. Biol. 42( 5): 681-689.
-Ishay, J. S. and Shimony, T. B. (1982) Temperature dependence electrical resistivity
of the hornet cuticle. J. Thermal Biol. 7: 91-94.
-Ishay, J. S. and Shimony (Benshalom), T. (1983) Electrical resistivity in cuticle
of Oriental hornet queen before, during and after hybernation: Evidence for
electronic conductance. PhysioL Chem. Phys. & Med. NMR 15( 4): 289-310.
-Ishay, J. S., Shimony (Benshalom), T., Lereah, Y. and Duby, T. (1982) Temperature
depen dence of electrical resistance of hornet and ant cuticle in low temperature:
Direct current measurements. Physiol. Chem. Phys. 14: 343-361.
-Ishay, J. S., Shimony (Benshalom), T. and Arcan, L. (1986) The biomineralization
in social wasps (Vespinae): The Presence of Statoliths. Scanning Electron Microscopy
IV. 1619- 1637.
-Ishay, J. S., BenshalomShimony, T., Weiss, D. and Kristianpoller, N. (1987)
Luminiscence properties of the Oriental hornet Vespa orientalis cuticle. Physiol.
Chem. Phys. & Med. NMR 19( 4): 283-294.
-Ishay, J. S. and Lim, S. M. E. (1990) Digging activity in the Oriental hornet
Vespa orientalis (Hymenoptera, Vespinae) is correlated with solar radiation.
J. EthoL 9( 12): 61-68.
-Ishay, J. S., Chernobrov, H. L. and Abes, A. H. (1990) Electric properties
of the hornet cu ticle: Voltage, current, resistance and capacitance and their
compliance to changes in tem perature. Physiol. Chem. Phys. & Med. NMR 22:
45-60.
-Ishay, J. S., Abes, A. H., Chemobrov, H. L. and Ishay, 1. (Z) and BenShalom,
A. (1991) Electrical properties of the Oriental hornet (Vespa orientalis) cuticle.
Comp. Biochem. & Physical 100( 2): 233-271.
-Ishay, J. S., BenshalomShimony, T., BenShalom, A. and Ktistianpoller, N. (1992)
Photo-voltaic effects in the Oriental hornet. J. Insect Physiol. 38( l): 37-48.
-Ishay, J. S. and Shmuelson, M. (1994) Symbiosis with a fungus produces the
colored stripes in social wasps. Physiol. Chem Phys. & Med. NMR 26( 3):
245-260.
-Ishay, J. S. and Shmuelson, M. (1996) Thermoelectric properties of the hornet
comb: A device for producing, transforming and storing electrical energy for
the entire colony. Physiol. Chem. Phys & Med. NMR 28: 41-54.
-Ishay, J. S. and Levtov, E. (1994) Sleep duration in hornets is influenced
by physical and chemical means. Comp. Biochem. Physiol. 109A: 567-573.
-Ishay, J. S., Pertsis, V. and Levtov, E. (1994) Duration of hornet sleep induced
by ether anesthesia is curtailed by exposure to sun or UV irradiation. Experientia
50: 737-741.
-Ishay, J. S. and BarenholzParfiry, V. (1995) Thermoelectric effect in hornet
silk and ther moregulation in hornets nest. J. Insect. Physiol. 4]( 9): 753-759.
-Ishay, J. S. and Litinetsky, L. (1996) Thermoelectric current in hornet cuticle:
Morphologi cal and electrical changes induced by temperature and light. Physiol.
Chem. Phys. & Med. NMR 28( l): 55-67.
-Jongebloed, W. L., Dunnebier, E. A., Albers, F. W. J. and Kalicharan, D. (1996)
Demonstra-tion of the stereocilia fine structure in the organ of Corti of the
guinea pig by field emission scanning electron microscopy (FEGSEM). Scan. Microsc.
10( l): 147-164.
-Joshua, H., Fischl, J., Henig, E., Ishay, J. and Gitter, S. (1973) Cytological,
biochemical and bacteriological properties of hemolymph and other body fluids
of Vespa orientalis. Comp. Biochem. Physiol. 45( B): 167-175
-Kalicharan, D., Jongeblord, W. L., Los, L. I. and Worst, LGF. (1992) Application
of tannic acid non coating technique in eye research: Lens capsule and cataractous
lens fibres. Bear electronenmikrosko Direktabb. Oberfl. 25: 201205. Ed. U. Ehrenwerth;
RA Remy Verlag, Munster (Germany).
-Kemper, H. and DOhring, E. (1967) Die sozialen Faltensespen Mateleuropas. P.
Parey, Berlin.
-Kittel, C.( 1968) Introduction to Solid State Physics. J. Wiley and Sons, New
York.
-Kristianpoller, N., Goldstein, 0., Litinetzky, L. and Ishay, J. S. (1995) Light
curtails sleep in anesthetized homets: extraretinal light perception. Physiol.
Chem. Phys. & Med. NMR 27: 193-201,
-Kruse, P. W., McGlaushlin, L. D. and McQuistan, R. B. (1963) Infrared Technology.
John Wiley & Sons, Inc. New York.
-Land, M. F. (1972) The physics and biology of animal reflector. Progr. BiophYs.
MoL Biol. 24: 75-106.
-Land, M. F. (1985) Optics of insect eyes. In: Comprehensive Insect Physiology,
Biochem istry and Pharmacology, Vol. 6, (G. A. Kerkut and L. I. Gilbert, eds),
Oxford, Pergamon, pp. 22-57.
-Locke, M. ( 1966) The structure and formation of the cuticulin layer in the
epicuticle of an insect. Calpodes ethlius (Lepidoptera, Hespiriidae). J. Morphol.
1/ 8: 461494.
-Matsuura, M. and Sakagami, S. F. (1973) A bionomic sketch of the giant hornet,
Vespa mandaninia, a serious pest for Japanese apiculture. J. Fac. Sci. Hokkaido
Univ. (VI Zool) 9: 125-162.
-Matsuura, M. and Yamane, S. (1990) Biology of the Vespine Wasps. Springer Verlag,
Berlin.
-Maycock, P. D. and Stirewalt, E. N. (1981) Photovoltaics. Sunlight to Electricity
in One Step. Brich House Pub. Co., Andover, Mass.
-Neville, A. (1975) Biology of the Arthropod Cuticle, Springner Verlag, Berlin.
-Rosenzweig, E., Fuchs, D. and Ishay, LS. (1985) Electrical resistance of hornet
cuticle: changes induced by xanthinesa statistical model. Physiol. Chem. Phys.
& Med. NMR 17( 4): 435-449.
-Rudall, K. (1963) The chitin protein complexes in insect cuticle. Adv. Insect
Physiol. 1: 257-313.
-Schaffer, J., Kramer, K. J., Garbow, J. R., Jacobs, G. S., Stejskal, E. O.,
Hopkins, T. L. and Speirs, R. D. (1987) Aromatic crosslinks in insect cuticle:
Detection by solidstate 13C and 15 N NMR. Science 235: 1200-1203.
-Shimony, T. B. and Ishay, LS. (1981a) Themoelectric (Seebeck) effect on the
cuticle of social wasps. J. Theor. Biol. 92: 497-503.
-Shimony, T. B. and Ishay, LS. (1981b) Pigment granules in the tegumental yellow
strips of social wasps: A scanning electron microscope study. Z. mikranat. Forsch.
95( 2): 310-319.
-Shimony (Benshalom), T. and Ishay, J. S. (1984) Electrical capacitance in hornet
integu-ment:
-Frequency, light and temperature dependence; possible PN junction effects.
Physiol. Chem. Ph vs. & Med. NMR 16( 4): 333-349.
-Spradbery, J. P. (1973) Wasps. Sidgwich At Jackson, London.
-Vecht, J. van der. (1957) The Vespinae of the IndoMalayan and Papuan areas
(Hymenoptera, Vespidue). Zool. Verb. Leiden, 34: 183.
-Vecht, J. van der. (1959) Notes on Oriental Vespinae, including some species
from China and Japan (Hymenoptera, Vespidae). Zool., Leiden, 36: 205-232.
-Watson, H. A. (1969) Electrical Conduction in Semiconductors. Devices and their
circuit application. 4: 6294. McGrawHill Inc., New York.
-Whitcomb, R. F., Shapiro, M. and Granados, R. R. (1974) Insect defense mechanisms
against microorganisms and parasitoids. In: The Physiology of Insecta, 2nd Edition,
(M. Rockstein, ed), Vol. V., Academic Press, New York, pp. 447-536.
-Wilson, E. O. (197 1) The Insect Societies. Belknap, Harvard, Mass.
-Ziegler, 3. and Harmsen, R. (1969) The biology of pteridines in insects. J.
Insect. Physiol.