Materials and Methods
Specimens of the Oriental hornet Vespa orientalis (Hymenoptera;
Vespinae) were collected from fields surrounding the Tel-Aviv metropolitan area
by a methodology previously described (Ishay, 1964). One-day-old hornets (i.
e., with a relatively soft, as yet untanned cuticle), ecloded from a comb which
was previously collected from a natural nest in the field, were anesthetized
with ether and then decapitated. The heads were rinsed briefly in 0.1 M cacodylate
buffer solution and then fixed in a mixture of 2% glutaraldehyde and 2% acrolein
in cacodylate buffer for 24 hours. Specimens were prepared for light microscopy
(LM), field emission electron microscopy (FE-SEM) and transmission electron
microscopy (TEM). For FE-SEM the specimens were next prepared according to the
tannic acid/ arginine/ osmium tetroxide non-coating technique (Jongebloed et
al., 1996). Dehydration with ethanol was followed by critical point drying (CDP)
in liquid C0 2 . SEM observations were carried out with a Jeol. FE-SEM, type
6301F, operated at 2-3 kV. Small portions of previously observed FE-SEM samples
were carefully oriented and subsequently embedded in Epon. Ultrathin sections
were poststained with uranyl acetate/ lead citrate and observed in a Philips
TEM, type CM 100, operated at 60 kV. Specimens for LM were Hematoxlyn Eosin
stained.
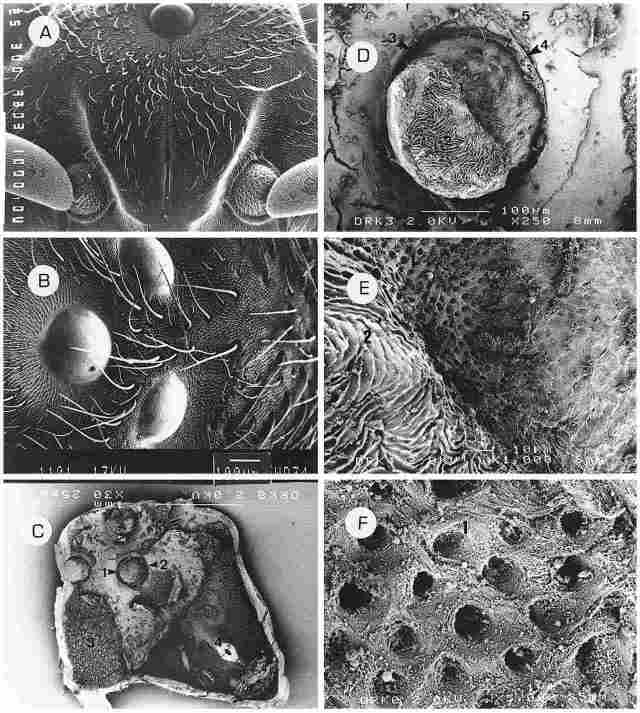
PLATE I: provides a general inner and outer picture of the upper anterior aspect
of the hornet's head. A: The median ocellus is seen at the top and proceeding
downwards is the coronal suture which extends between the two halves of the
frons plate. The ocellus is located on the vertex. Bar= I Ám. B: All three ocelli
can be seen, arranged at equal distances apart (with the median ocellus seen
here on the left). The cuticle around each ocellus is recessed forming a sort
of broad canal or moat. Around each cornea there is a narrow strip devoid of
setae or sensilla. Between the ocelli there are long or short bristles. The
surface bearing the ocelli forms a trigonal pyramid whose apex is blunt. Bar
= 100 Ám. C: A general view of the frons and vertex regions from the interior
side of the cuticle. At the base of each ocellus there is a canal (1) which
girdles it and around the canal there is a circular crista (2). The surface
between the ocelli as well as to the right, left and below them, including also
the frons region, is covered with ciliary cells (3). Also visible is the conus
(4) which comprises the inner side of the depression designating the coronal
suture. Bar = I Ám. D: The base of the ocellus from its interior, with the fenestrated
lamina at the base (1). Around this area the cuticle has an areolar appearance
(2). As already mentioned, around the base of the ocellus there is a moat-like
canal (3)-the so-called periocellar sulcus. This canal is enveloped by the periocellar
membrane (4). Around the entire ocellus there is a layer of ciliary cells (see
below) and the membrane covering these cells is interspersed with otoliths (5).
Bar= 100 Ám. E.-An enlargement of the base of the ocellus, showing the fenestrated
lamina (1) and the areolar cuticle (2). Bar = 10 Ám. F. Further enlargement
of the fenestrated lamina showing perforations (1) which are the outlets for
the dendrites emerging from the ocellus and comprising the ocellar nerve (O.
N.). The diameter of these outlets is about 2 Ám and they appear of uniform
shape and size. Bar = 1 Ám.
Navigation experiments
Hornet combs collected in the field following ether anesthesia (Ishay,
1975) were transported to our laboratory. Workers ecloding from these combs
were gathered on the day of eclosion and accustomed to fly from a fixed point
within their artificial breeding box (ABB). Three days later they were released
from increasingly greater distances in order to determine the extent of their
spatial orientation and their navigational capability. The orientation of these
hornets was then compared with that of hornets whose ocelli were coated with
Tippex and with that of hornets whose compound eyes (ommatidia) were similarly
coated. Complete details on this experiment are to be published separately (Kirshboim
and Ishay, in preparation).
Results
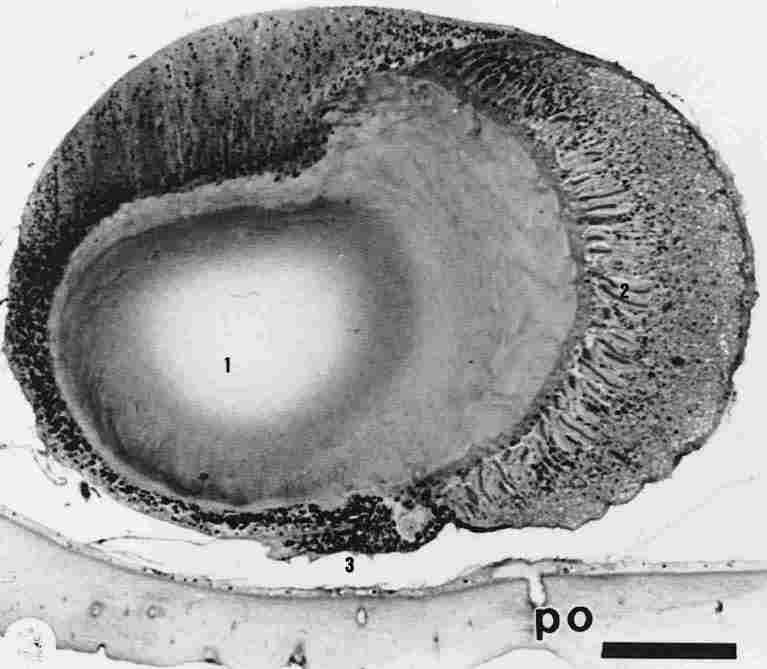
FIGURE 1. Cross section through one ocellus (median). One can see (1) the lens,
(2) retinula, (3) and pigment granules. PO = peripheral photoreceptor in the
cuticle. Bar = 100 Ám.
A. The Morphological Studies Plates I, II, III and Plate X, Figure C represent
SEM sections, Plates IIA and IIB, LM sections and Plates IV-X. An ocellus is
an intracuticular organ built in the shape of an upsidedown cone whose base
faces out and contains a convex corneal lens, while its truncated apex faces
inwards and from it extends the ocellar nerve. Diameter of the cornea is about
400 Ám and that of the blunt apex is about 250 Ám (see Plate 1, Figures A-D
and Plate X, Figure Q. Each ocellus is situated on the flat cuticle of the vertex.
The domeshaped corneas of the ocelli protrude outwards at an orientation of
90 0 to one another, with the distance between the two lateral ocelli being
330 Ám while that between them and the median ocellus is 250 Ám (Plate I, Figure
B). From each lateral ocellus another organ can be traced extending in a straight
line. This organ is surrounded by circles of setae and the entire area is recessed.
Proceeding diagonally from this organ through the lateral ocellus, we end up
at the median ocellus (Plate 1, Figures A, B). Proceeding from the median ocellus
there is first, on the vertex, a glabrous 'line' which later on, upon the frons,
becomes a groove called the sutura coronalis (S. C.) (Plate I, A). The comea
of the ocellus is smooth and is surrounded by a ring of cuticle which is also
smooth and devoid of any setae (see Plate 1, Figures A, B and Plate III 3 a,
b, d). Under the cornea there is one lens in each ocellus (see Figure I and
Plates IIA and III). In its internal end, the ocellus is also bounded by cuticle
through which traverse the 'necks' of the monopolar cells. Each 'neck' links
the external portion of the monopolar cell, which bears the sensory component,
with the internal portion which consists of the body of the cell and the axon
that extends from it (Plate I, Figures C-E). We deem it proper to designate
this region as the 'perforated cuticle. ' The perforations in this region are
of uniform diameter 2 Ám. Below the perforated cuticle area the cuticle acquires
an areolar configuration. Such structure of the cuticle is unique for the ocelli
and is not encountered anywhere else in the hornet body. This cuticular configuration
enables rapid flow of hemolymph and contact of hemolymph with extensive surfaces,
both of which ensure thermal homeostasis of the ocellus (Plate I, Figures D,
E). Around each ocellus there is a deep, moat-like canal that separates the
ocellus from the surrounding cuticle. The canal, in turn, is surrounded by the
periocellar membrane, which is a twinlayered membrane whose inner layer enwraps
the ocellus, while the outer layer enwraps the cuticular frame around it. Between
the two layers there is a gap which, most probably, functions as a passage for
hemolymph (periocellar sulcus) (Plate I, Figure D; Plate IIA, Figures b, e;
Plate III, Figure A; and Plate X, Figure D). Around the ocellus there is a layer
of ciliary cells which is covered by a membrane (Plate III, Figure A, I and
Plate X, Figure F), and above this membrane there are otoliths (Plate 1, Figure
D, 1). Consequently the mentioned membrane is designated by us as the otolithic
membrane. Underneath the otolithic membrane there is a continuous layer of tall
ciliary cells whose cilia proceed inwards to come in direct contact with the
otolithic membrane. Underneath this layer of ciliary cells there is an additional
layer of ciliary cells but these cells are rather shorter and their cilia are
directed toward the cuticle, that is, they are orientated 180 0 away from the
cilia of the previous layer. The cilia of the latter layer come in contact with
the basal membrane of the cuticle (Plate III, Figures AF, Plate X, Figure F).
The ciliary cells extend up to the periocellar sulcus and gird the cuticle in
this region, which forms an annular crista around the sulcus and therefore is
designated as the periocellar crista. The ciliary cells are numerous and arranged
in groups, with each group separated from the others by a septum (Plate III,
Figures B, E, 2). Each group is comprised of 6-8 cells, with the diameter of
an entire group being about 40 Ám. The separatory septum appear to be higher
than the cilia, which suggests that in the intact hornet it comprises a sort
of tubule that probably contains a special fluid (i. e., different in ionic
or other respects from the hemolymph). The cilia do not cover the rear surface
of the ocellus. The surface shown in Figure B (Plate III) boasts numerous groups
of ciliary cells and from other figures we clearly see the cilia covering surfaces
of the vertex around the ocelli and the frons Plate I, Figure Q. Throughout
the surface are distributed perforations of pores of about 10 Ám in diameter,
which are the internal openings of the peripheral photoreceptors (Plate III,
Figure C, 1). In Plate III (Figure F), one can see magnified cilia (1), with
strings clearly interconnecting between pairs of cilia (i. e., tip links) (2).
Upon these interconnecting strings can occasionally be seen a ballshaped, minute
weight (3). On TEM (Plate X, Figure F), one sees cilia (1), with their basal
body (2), that are directed toward the cuticle, that is, that contact the basal
membrane of the cuticle (3). In a sagittal section, through the center of an
ocellus one can see the envelope enwrapping the entire ocellus. This envelope
is composed of two layers of membranes the outer one the thicker of the two,
contains an abundance of pigment granules (1), while the inner one is thinner
and with a sparser amount of pigment (2) (Plate IV, Figures A-C).
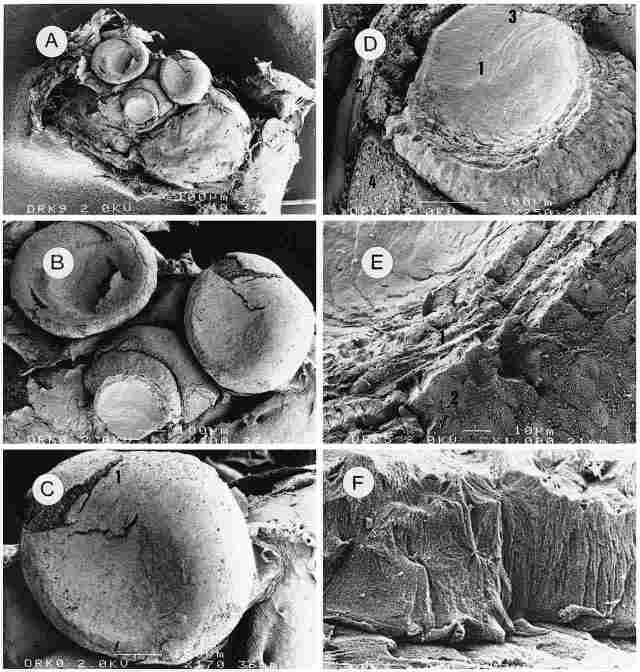
PLATE II: The cornea and lens Figure A: Outside view of the three ocelli that
had been dried during their preparation for SEM micrography. In the median ocellus
(here seen at bottom) the cornea has been removed to reveal the lens. Neighboring
tissues are also evident. Bar = 100 Ám. Figure B: As the previous picture but
enlarged. In the two upper ocelli the liquids have evaporated out of the lens
owing to drying of the preparation during its processing and consequently the
cornea appears sunken. Bar = 100 Ám. Figure C: The lateral (left) ocellus under
further magnification. Part of the cornea ( I ) has split open, revealing the
bodies of corrneogenic cells. Bar = 100 Ám. Figure D: Enlargement of the lens
(1). One can see the envelopes (2) and also, above the lens, an opening of the
trachea (3), and sectioned corneogenic cells (4). Bar = 100 Ám. Figure E: Enlargement
of the lens, but this time we see a transverse aspect of the layered structure
of the lens (1) and also the envelopes of the lens (2) which are composed of
several layers, probably 4. The envelopes and lens appear to be devoid of any
cellular structure, which suggests that they are transparent to light. Bar =
10 Ám. Figure F. Enlargement of the outer layer of the lens envelopes. Here,
too, there is no evidence of any cellular struc-ture. Bar = 1 Ám.
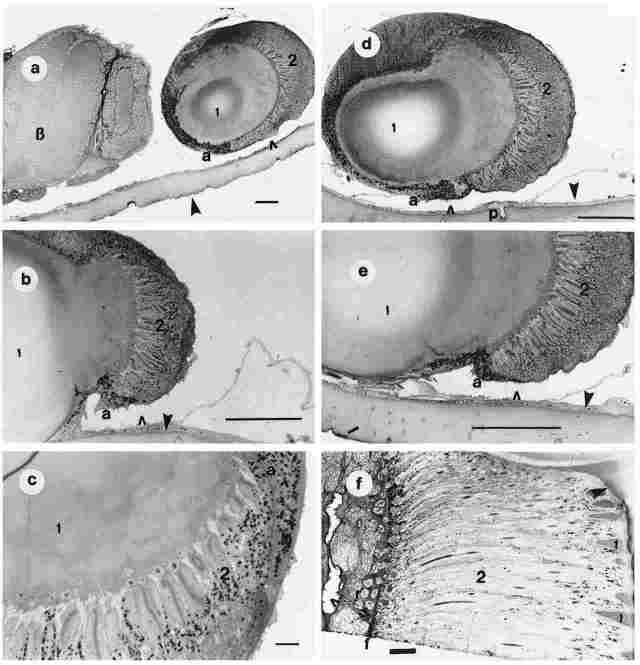
PLATE IIA. Figures a-e: Light microscopy of the ocellus in cross sections. Figure
a: I-section through the lens; 2-the retinular layer; a-the adventitia of the
ocellus; hollow arrow-periocellar sulcus; solid aroow-cuticle; B = brain. Bar
= 100 Ám. Figure b: I-lens; 2-retinular layer; a-adventitia; hollow arrow-periocellar
sulcus; solid arrow-basal membrane and cuticle. Bar = 100 Ám. Figure c: I-lens;
2-refinular layer; a-adventitia. Bar = 100 Ám. Figure d: I-lens; 2-retinular
layer; a-adventitia; p-photoreceptor; hollow arrow-periocellar sulcus; solid
arrow-basal membrane and cuticle. Bar = 100 Ám. Figure e: I-lens; 2-retinular
layer; a-adventitia; hollow arrow-periocellar sulcus; solid arrow-basal membrane
and cuticle. Bar = 100 Ám. Figuref: cross section of an ommatidium for comparison
purposes. 1-the conus; with the protruding comea also visible (arrow); 2-retinulae;
near the 'f' is a transverse section of a group of reticulae in the compound
eye; hollow arrow-hemolymphatic space; in base of fenestrate membrane. Bar=
100 Ám.
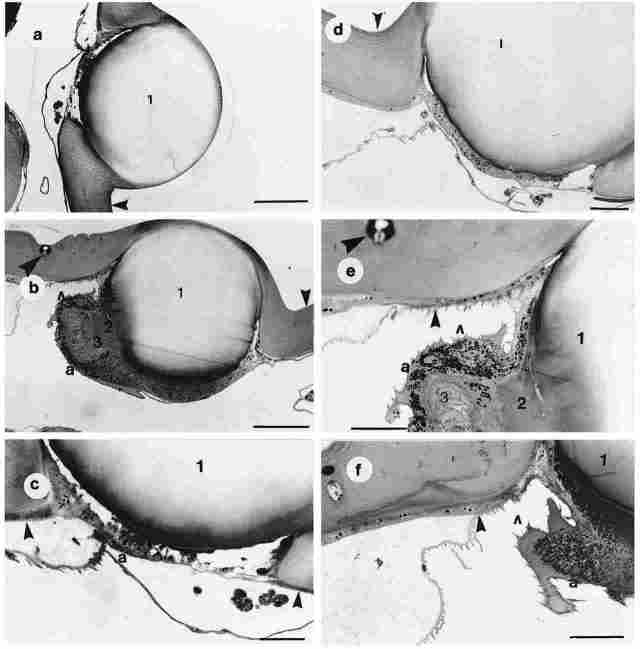
PLATE IIB. Figures a-f., Cross section through the cuticle and the comes of
one ocellus. Figure a: I-the cornea; arrow-the cuticle around the comea. The
comea is covered by cuticle (a single transparent layer of epicuticle). Bar
= 100 Ám. Figure b: I-the cornea; 2 the lens; 3-the retinula; a-hemolymphatic
space; small arrow-the cuticle; big arrow the paraocellar organ. Bar = 100 Ám.
Figure c: I-the cornea; a-hemolymphatic space; arrow-the cuticle and its basal
layer. Bar 100 Ám. Figure d: 1 -the cornea; arrow-the cuticle around the lens.
Bar = 50 Ám. Figure e: I-the cornea; 2-the lens; 3-the retinular layer; a-the
adventitia; small arrow-the hemolymphatic space; small dark arrow-the cuticle
and its basal membrane; large dark arrow the paraocellar organ. Bar = 50 Ám.
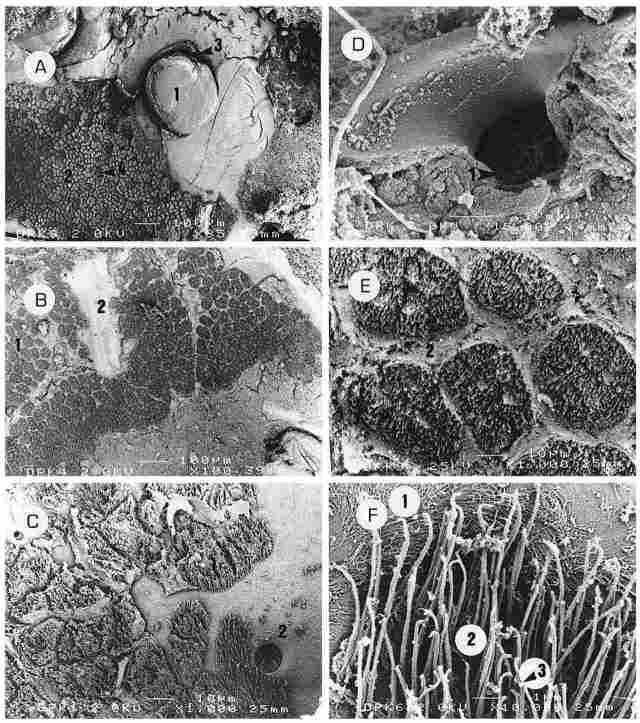
PLATE III : An inside view of the ocelli and their environs. A: The inner aspect
of two ocelli (1), with ciliary cells around them (2). These ciliary cells cover
the entire surface up to the periocellar sulcus (3) and between them can be
seen cells of peripheral photoreceptors (PRs) (4). Bar = 100 Ám. B: The entire
inner surface to be overlaid with ciliary cells (1) arranged in aggregates that
are distinctly separated from one another by fibrous tissue connecting to the
cuticle (2). Bar100 Ám. C: The ciliary cells in greater magnification (1) and
also the inlet of a PR (2). In this region, the PRs are quite numerous, albeit
not seen in the picture. Bar = 10 Ám. D: For comparison purposes, the outlet
of a PR. On the outside is seen the membrane that covers the PR (1) as it is
recessed on the underside of the exocuticle. Bar = I Ám. E: Aggregates of ciliary
cells (1) separated by partitions of fibrous tissue (2). Each aggregate has
a diameter of 35-40 Ám and seems to be comprised of cilia that arise from 7-8
cells. These aggregates acquire a rounded to hexagonal shape. The 'container'
housing each aggregate is sealed in the intact, live hornet, and needs to contain
a fluid of certain ionic composition. Bar = 10 Ám. F: An enlargement of the
cilia (1) with a number of interconnections between them (2). Upon these interconnections
a ball-shaped, minute weight can be seen (3). The width of each cilium is about
0.1 Ám or less and its length is 5-7 Ám Bar = I Ám.
Around the envelope one can see a hemolymphatic space enwrapped by a typical
basal membrane (3). This space is actually the distal end of the periocellar
sulcus (Plate X, Figure D). Underneath the cornea there is a layer of corneogenic
cells which are completely devoid of pigment. These cells possess an elongated
nucleus situated at the base (Plate IV, Figures A, B). Below the nucleus the
corneogenic cell extrudes a process that penetrates deep between the retinular
cells so that the two retinular cells comprising each rhabdom are separated
by this extension of the corneogenic cells which reaches right up to the distal
end of the rhabdom (Plate IV, Figure C, 4). The retinular cells are located
deeper than the corneogenic ones and they are about 90 Ám long. The retinular
cell is actually the monopolar cell and it is composed of an external and internal
component. Between the two components is located the neck of the cell, whose
width is half that of the cell in its other parts. The neck of the cell passes
through a region of the cuticle that has an areolar configuration. This areolar
region is domeshaped, about 4-5 Ám in thickness and forms a layer that separates
between all the distal, sensory elements and the proximal, neural elements of
the ocellus. Barring the neck part, the cell through its entire length is rather
uniformly thick, measuring about 4 Ám in width.
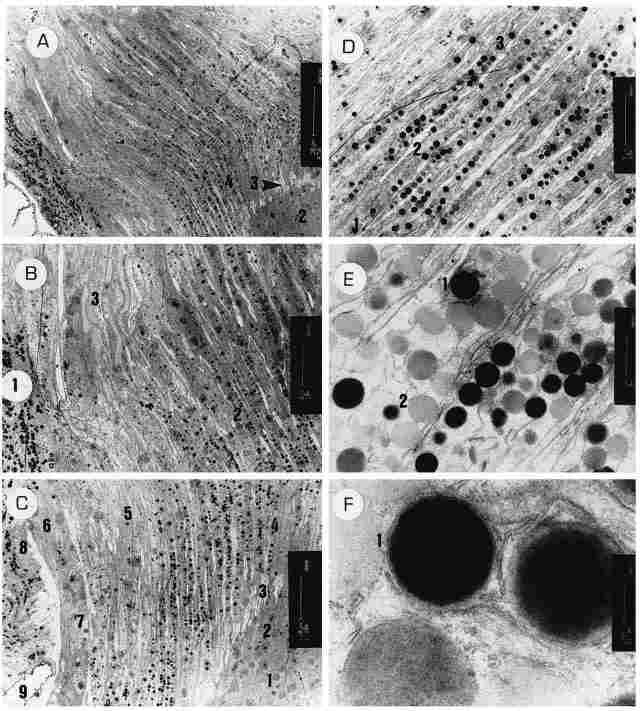
PLATE IV (opposite page): Longitudinal sections of the ocellus.
Figure A: A sagiral section through an ocellus. On the left of the figure can
be seen a segment of the ocellar envelope (adventitia) containing a plethora
of pigment granules ( 1) (apparently melanin) inside. For the most part, the
section passes through the retinular cells. In the right corner can be seen
a region populated with monopolar neurons (2) and the transition from the lightcolored
areolar region (3) to the region of the rhabdom (4). Bar = 20 Ám Figure B: On
the left a portion of the ocellar adventitia with much pigment (1). Most of
the figure shows the retinular cells in their distal parts (2), while on top
one can see the comeogenic cells and their nuclei (3). Bar = 10 Ám. Figure C:
On bottom right (1) the bodies of the monopolar neurons, with their nuclei (2)
which are slender and about 8 par in length, and the 'necks' (3) of these cells
that pass through the areolar region to make up the rhabdom (4) immediately
distal to it. Indeed, the highest concentration of pigment is encountered distal
to the region of the rhabdoms. On the left of picture (3) can be seen corneogenic
cells that contain very little pigment and have nuclei that appear very elongated
(about 20 pm) and bear a nucleolus at the center. Further to the left (6) can
be seen the ocellar adventitia whose cells boast a large nucleus (7) with intranuclear
inclusions. At the left margin of the picture can be seen the envelope cells
with an abundance of pigment (8). On bottom left (9) we see a space for hemolymph.
Bar = 10 Ám. Figure D: The transition zone between the retinular cells and the
corneogenic cells. Every two retinular cells are coupled tightly in the region
of the rhabdom (1) but break apart distal to the rhabdom to allow the interposition
of a long (and translucent) process of a corneogenic cell which extends up to
the rhabdom proper (2). In their distal part the two retinal cells comprising
the rhabdom are separated from each other by the interposed process of a corneogenic
cell (3). Bar= 5 Ám. Figure E: An enlargement of the retinular cells distal
to the rhadbom. There are numerous pigment granules some electron-dense ( I
and others of a lighter color (2). Width of the retinular cells in this region
is about 2.5 Ám. Bar I Ám. Figure F. A further enlargement of the pigment granules,
showing clearly that they are enwrapped by a membrane composed of endoplasmic
reticulum (1). Bar= 200 Ám
In the bottom third of the external component is located the rhabdom (Plate
V, Figure D), while the upper two thirds contain a large amount of pigment granules
(Plate V, Figure Q. The internal component contains the cell nucleus (Figure
Q and from this component also extends the axon which conjoins the ocellar nerve.
To recap, the following is a full description of a retinular cell. Starting
with the external component, its distal part contains a large quantity of pigment
granules of differing electron density (Plate IV, Figure D, 1,2). Each pigment
granule is enwrapped in a delicate envelope which probably originates from the
endoplasmic reticulum. In their distal part the two retinular cells comprising
the rhabdom are separated from each other by the interposed process of a corneogenic
cell (Plate IV, Figure D, 3).
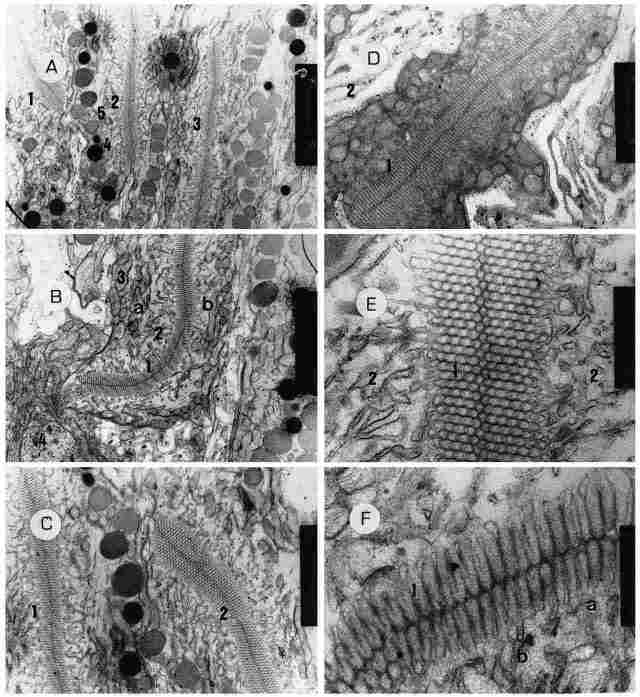
PLATE V: The retinular cell in the rhabdom level. A: Three rhabdoms arranged
more or less in parallel (1,2,3) and 5 pm apart, which is tantamount to the
width of a single retinular cell. The picture also shows a series of pigment
granules, some of which are electron-dense (4) while others are lighter in color
(5), as well as numerous other intracellular organelles (see below). Bar = 2
Ám. B: A rhabdom (in center) (1) from the proximal end of the ocellus~ this
rhabdom is composed of two retinular cells (a, b), and around it there is a
'sleeve' of amorphous acellular matter containing remnants of membranes that
had undergone degeneration (2). Outside these there is a very large concentration
of mitochondria (3) near the outer cell membrane. In the lower part of the picture
can be seen the transition from the arcolar region to the main body of the receptor
cell, with part of the nucleus of the monopolar cell also visible (4). Bar =
2 Ám. C: Another view of two rhabdoms in the distal part of the ocellus (1,2).
The configuration here is as depicted in Figure B except that Figure D: The
base of a rhabdom (1) in greater magnification. Again we can see the structure
depicted in Figure B. Around the retinular cell is seen the areolar region (2).
Bar = 1 Ám. E: A magnified section of a rhabdom (1), on both sides of which
can be seen remnants of retinular membranes (2) at various stages of degeneration.
Bar= 200 nm. F. The same picture as in the previous figure, but here can be
seen the folds of the rhabdom (1) in which the mem-brane forms, elongates (a)
and extends into the cell (b). Bar = 200 nm.
As for structure of the proximal part of the external component this can be
seen in detail in Plate V. In this proximal part of the external component we
find the rhabdom, which is actually composed of the plasmalemma of two conjoined
reticular cells. Around the rhabdom there is a region composed of amorphous
matter which contains remnants of old or used membranes traceable to the rhabdom.
Around this area, in turn, there is a very high concentration of mitochondria,
while in the layer near the external membrane of the retinular cell one encounters
also pigment granules, albeit in significantly smaller number than in the distal
part of the retinular cell (Plate V, Figures A-C). In the lower portion of the
rhabdom (actually beneath it) one discerns the neck of the cell which passes
through the areolar region of the ocellus that encircles also the base of the
cell. The rhabdom measures about 30 Ám in length and is straight or upright
in most cases. As pointed out, the rhabdom here is a special structure formed
from the plasmalemma of two conjoining retinular cells and having a width of
about 0.5 Ám. Each retinular cell thus contributes a membrane and the distance
between the two membranes is uniformly about 100 nm. Between these twin membranes
can be found microfilaments and microtubuli whose function is to safeguard the
structure and ensure collection and disposal of metabolites (Plate VII, Figures
D-F). In the region of the rhabdom, the membranes between the two retinular
cells actually conflate into a single membrane to create a fullfledged desmosome
through which microfilaments intercross from one retinular cell to the other
(Plate VII, Figure F). Cross section through an ocellus reveals disparate layers
of the ocellus owing to its dome shape. Thus, in the center are visible the
necks of the refinular cells which traverse the arcolar region and around them
one sees the region of the rhabdoms which, in turn, are surrounded by distal
parts of the retinular cells (Plate VI, Figures A-B). Noteworthy, in such cross
section is the fact that each rhabdom is comprised of two retinular cells and
that the width of the rhabdom here is about 45 Ám (Plate VI, Figures CE). Oxygen
supply of the retinular cells is achieved through the tracheae which interpenetrate
between the cells. Thus, each duplex of retinular cells is separated from neighboring
duplexes by intercellular matrix containing extensive spaces for hemolymph.
Yet, we need to remember that in their more distal parts, the two retinular
cells of a duplex are separated from each other (Plate VI, Figure F). In fact,
the distal parts of the retinular cells are seen to greater advantage in Plate
VIII. In Figure A of this plate, one can see pigment granules (1) that are about
600-800 nm in diameter. Also visible in Plate VIII are a few mitochondria (Figure
B) and endoplasmic reticulum (Figures B, C, 2). Noteworthy is the fact that
in this part of the cell there is no tight junction between the retinular cells,
that is, the membranes of neighboring retinular cells are quite apart. Note
also the large number of ribosomes (as represented by the granulation along
the endoplasmic reticulum, 3). The inner envelope is comprised of a layer of
cells possessing a large nucleus and endoplasmic reticulum in considerable amount
(Figures D, E). Also visible within the cytoplasm are numerous ribosomes, as
well as organelles encased in multiple membranous envelopes, that resemble the
multilamellar bodies occurring in the fat body of insects (Figures E, F). The
diameter of these organelles ranges between 400-800 nm. Additionally, one can
see near the MLB-like organelles also other organelles, possessing numerous
vesicles and accordingly resembling multivesicular bodies (MVB) (Figures D-F).
In fact, wherever there are MLB-like organelles, there are also MVB-like organelles.
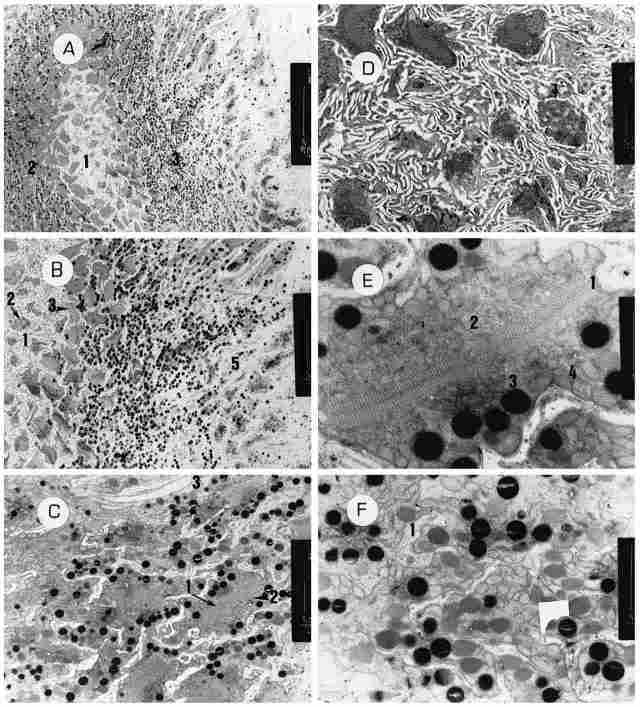
PLATE VI : Transverse sections through the ocellus A: A cross-section through
the base of the ocellus. In the center we see the areolar region (1) and around
the rhabdoms (2) which, in turn, are surrounded by the distal ends of the retinular
cells which contain pigment granules (3). Bar = 20 Ám. B: As the previous figure,
but further enlarged. From left to right, one can see the following: the areolar
region (1) and in it the 'necks' of the retinular cells (2), the bases of the
rhabdoms (3), the distal ends of the retinular cells (4) and the area of corneogenic
cells (5). Bar = 10 Ám C: A cross-section through the rhabdoms. Several of the
latter are visible: pigment granules ( I ) as well as the retinalar cells comprising
them (2) and a trachea (3). Width of each rhabdom is 4-5 Ám. Bar = 2 Ám. D:
A cross-section through the areolar region, showing 'necks' of retinular cells
( 1) and also the bases of several rhabdoms. Bar = 2 Ám. E: An enlargement of
the base of one rhabdom. In the center one sees the membranes of the rhabdoms
(1) and around them amorphous intracellular matter (2) with remnants of membranes
and a few pigment granules outside them (3). There is an uninterrupted array
of mitochondria around the pigment granules (4). Bar = I Ám. F: A cross-section
through retinular cells located just distal to the rhabdoms. There is an abundance
of pigment granules and mitochondria. Between the cells are seen the extensions
of the corneogernic cells (1). Bar = 2 Ám.
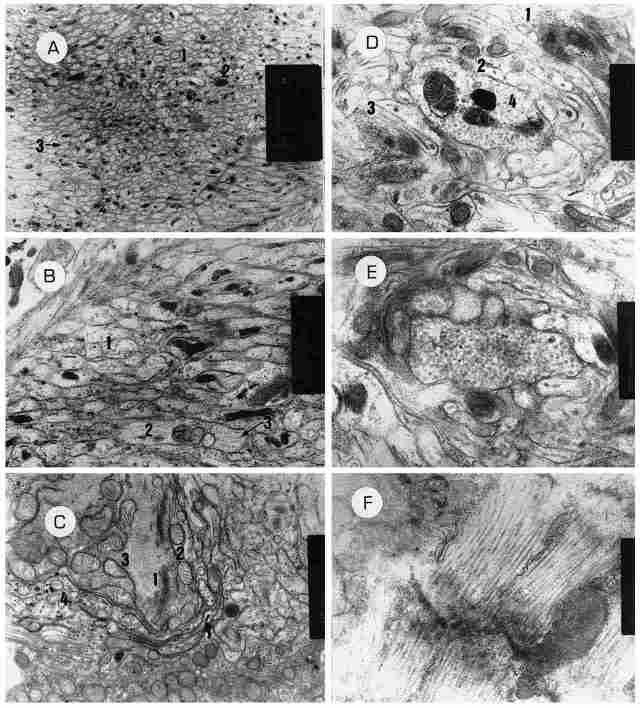
PLATE VII: The axon, and the rhabdomal membranes. A: A cross-section through
the axons (1) which emerges from a monopolar neuron. In fact, it shows numerous
axons crosswise. The large black spots (2) are pigment granules while the small
black dots (3) are microfilaments cut crosswise. Bar = 2 Ám. B: The same cross-section
at greater magnification. A number of axons can be seen cut crosswise ( 1) while
other axons are cut lengthwise (2). Also visible are the neurofilaments (3).
Bar= 1 Ám. C. A section through the base of the axon that emerges from the monopolar
neuron. In the center of picture (1) there is an array of Golgi bodies and around
them numerous mitochondria (2) and rough encloplasmic reticulum (3) which, in
turn, is surrounded by glia cells (4). D: A cross-section through the rhabdomal
membranes, One can see several membranes ( L2,3) and inside them, microtubules
and microfilaments (4). At center there are several bodies. Between the folds
of the membranes are visible regions of cytoplasm containing mitochondria and
cytoplasmic inclusions. Bar= 500 nm. E: Likewise a cross-section but through
the fold of one rhabdomal membrane. Here we see pockets in the membrane whose
function is unclear. Bar = 500 nm. F: A sagittal section through a rhabdom.
One can see the folds of the rhabdomal membrane. The dark line running across
the folds is the membrane that separates between the two retinular cells that
comprise the rhabdom. Length-wise are seen the folds of the rhabdom. Note that
some filaments are passing through the membrane. Bar= 500 nm.
From the perikarion there extends an axon toward the protocerebrum. All the
axons unite to form the ocellar nerve. Each axon has a diameter of 0.25-0.50
Ám. Within the axon can be seen neurofilaments and a few pigment granules (see
cross-sections in Plate VII, A and Plate X, A as well as longitudinal section
in Plate VII, B). At the base of the axon are seen numerous mitochondria and
a golgi apparatus (Plate VII C. The perikarion is endowed with a large nucleus
bearing intranuclear inclusions, while its cytoplasm reveals relatively small
pigment granules (Plate VIII, A, B). Between the perikarions there are glia
cells which are supportive both structurally and metabolically (Plate IX, A).
Within the envelopes there are glia cells of the envelope, some of which are
with adipose accretions, and these cells too are characterized by forming septate
junctions with the retinular cell (Plate IX, D-F). Within the nuclei of the
latter one occasionally detects nucleoli as well as intranuclear inclusions.
Experiments on Navigation
1. Hornets with covered ommatidianone of the hornets whose compound
eyes (ommatidia) were coated with Tippex was able to return from a distance
of 100 meters. When the artificial breeding boxes (ABBs) housing the hornets
were opened in broad daylight, the hornets inside did not remain on the ground
but rather rose somewhat, as if attempting to fly, but none of the 15 'eyecoated'
workers succeeded in leaving the site of their liberation or returning to their
original ABB to which they have undergone imprinting (i. e., to the starting
position). Similar behavior was obtained in dim light.
2. Hornets with covered ocelli when released in dim light conditions (700 Lux
or less) only 7 out of 20 (35%) 'ocellicoated' hornets made it back (to their
starting position) in contrast to 14 out of 19 (74%) control (unTippexed) hornets.
Those who managed to return require more time to perform their task than the
test hornets, some did return only on the next day. These results repeated themselves
upon subsequent trials carried out during the active season of the hornets
Discussion
The ocellus is an intracuticular organ, that has a cornea, which is
part of the cuticle, comprised of translucent and convex epiexo-and endocuticle.
Underneath the cornea is located one lens common to all the retinular units
(see Plate II, Plate IIA, Figures b-e). The lens of the ocelli is homologous
to the comus of the ommatidium. Underneath it are located comeogenic cells,
which are analogous to the hypodermal cells of the cuticle. From each corneogenic
cell arises a centripetalic extension which penetrates between a pair of retinular
cells and reaches the rhabdom formed between the two cells. The reticular layer
occurring in the region of the neck of the cells is analogous to the bypocuticle
and therefore what is located between it and the comea is the intracunticlar
layer. Surrounding the ocellus is an envelope containing numerous pigment cells
which comes in contact with hemolymphatic space created by the periocellar sulcus.
The envelope, in fact, intervenes between the hemolymph and the fluid which
permeates the various parts of the ocellus and this, perhaps, justifies coinage
of the term ocellar endolymph which probably has an electrolytic make up differing
from that of the hemolymph in other parts of the body. The glia cells within
the envelope (Plate IX, C-F) apparently function also as barriers between the
two lymphatic compartments. It seems very important to consider the fact that
the electrolytic makeup and the osmotic pressure need to be of a constant nature,
especially insofar as the retinular cells undergo continuous depolarization,
so that a rapid repolarization is enabled. The envelope of the ocellus is composed
of two layers an inner and an outer one. The inner layer houses the glia cells
just mentioned above, while the outer layer houses pigment cells, probably containing
melanin, which form the pigment within the melanosomes (Plate VIII, D-F). We
conjecture that the melanosome appears initially as a multilamellar body (MLB)
which gradually fills up with melanin (Plate VIII, F) by the metabolism of tyrosine.
As is known, the fatbody of insects also contains intracellular organelles that
are designated as MLB and are important in tyrosine metabolism (Locke, 1984).
In the ocellar envelope, next to each cell showing the MLB, we find a multivacuolar
body. We are uncertain as to the role of the latter but suspect that there may
be some metabolic connection between the two (Plate VIII, D-F).
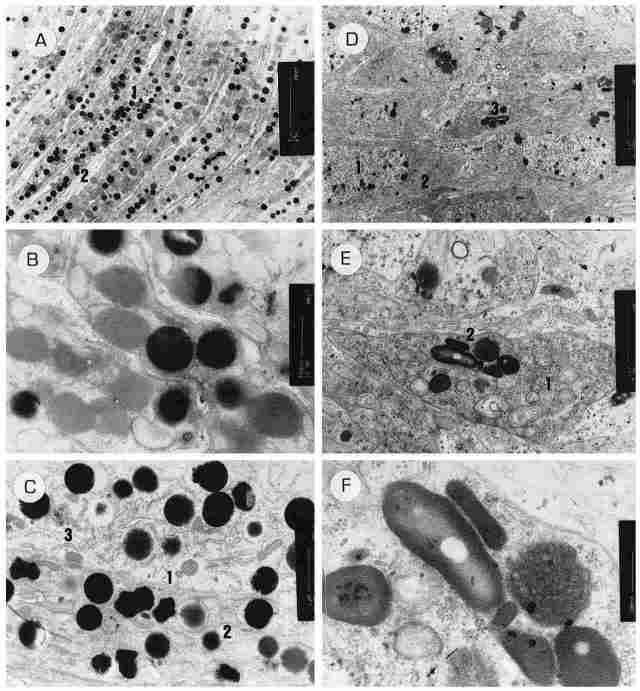
PLATE VIII: The envelopes and the retinular cells and the distal segments. Figure
A: Retinular cells distal to the rhabdoms. In them there is a large number of
pigment granules (1) with pro-cesses of corneogenic cells (2) interpenetrating
between each two retinular cells. Bar =5 Ám. Figure B: Two extensions of corneogenic
cells and around them, within the retinular cells, there we pigment granules
of either high or low electron density. Bar = 500 nm. Figure C: Two retinular
cells enlarged can be seen. One can see a separate membrane of each cell (1),
pigment granules (2), few mitochondria and rough endoplasmic retinulum (3).
Bar = 1 Ám. Figure D: An array of cells from the inner layer of the ocellar
envelopes. One can see cells with large nuclei (1), rough endoplasmic reticulum
(2) and intracytoplasmic inclusions (3). Bar= 1 Ám. Figure E: An enlargement
of the previous picture showing a cell from the envelope whose cytoplasm is
rich in ribosomes (1) and intracytoplasmic inclusions (2), the latter probably
comprising pigment granules in the process of formation. Bar = 1 Ám. Figure
F: The cell from the previous picture at greater magnification. One now sees
that the intracytoplasmic inclu-sions are built from envelopes of concentric
membranes resembling a lamellar body and with amorphous matter inside. Close
to these inclusions there is a round body with numerous vesicles inside. This
is a multivesicular body (MVB). Bar = 200 nm.
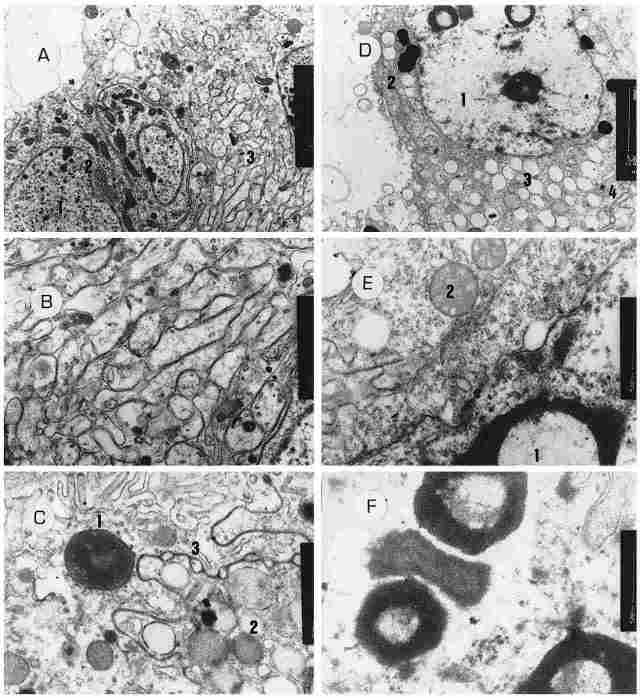
PLATE IX: The outer envelope of the ocellus. Figure A. Cells in the outer envelope
of the ocellus. A number of cells are visible. all show nuclei (1), a Golgi
apparatus (2) and numerous membranes whose role or origin are unclear (3). Bar
= 2 Ám. Figure B: A greater magnification of the same membranes. Bar = 500 nm.
Figure C: A cross-section through a glia cell located in the envelope surrounding
the ocel( us and belonging to the inner layer of the envelope. One can see the
nucleus of the cell (1) and also the membrane that separates between this cell
and the neighboring cell which contains pigment granules (2). Within this separatory
membrane one can see circles (3). Such structure is typical for the septate
junction between glia cells and other cells. Har = 500 nm. Figure D.-Another
glia cell within the inner layer of the okcellar envelope. We can see the nucleus
of the cell ( 1), which is clear but with intranuclear inclusions (possibly
representing viral invasions). Also discernible is a well-developed Golgi apparatus,
(2) and cytoplastic vacuoles (3). On bottom right can be seen the cell membrane
with septate junctions (4). Bar = 2 Ám. Figure E: The same cell at higher magnification.
We now see nuclear inclusions (1) and several rmtochondria cut transversely
(2). Bar = 500 nm. Figure F: Further enlargement of the cell's nucleus. The
intranuclear inclusions are discernible. Bar = 500 nm.
Interestingly, in these cells there are also ribosomes that are probably connected
with local polypeptide synthesis. Several investigators are of the opinion that
the melanin forming cells, too, are a type of glia cell which has specialized
in the creation of pigment (Baumann, 1975; Tracopoulus et al., 1981; Saint Marie
et al., 1984). Generally speaking the distribution of melanin in the ocellus
is primarily in the outer envelope and in the retimalar cellsaround the distal
part of the rhabdom. However, even around the proximal part of the rhabdom there
are a few pigment granules and these can be found also inside the perikarion
and even in the emerging axon. In the compound eye, in comparison, the pigment
granules are located at the most proximal (basal) part of the rhabdom (see Plate
IIA, Figure f). We presume that the pigment in the region is also produced within
the glia cells. In contrast, the layer of corneogenic cells is entirely devoid
of pigment granules. Which hormones are responsible for this situation is presently
unknown. The pigment granules within the retinular cells are enwrapped in a
thin membrane probably deriving from the smooth endoplasmic reticulum. The pigment
granules vary in their electron density, probably owing to a breakdown or catabolism
of melanine in the course of neutralization of freeradicals. The role of melanin
in the ocellus is probably to prevent the passage of light rays from one rhabdom
unit to another. The pigment in the region of the perikarion is responsible
also for the neutralization of free radicals which could be formed as a result
of light penetration into the region. The free radicals around neurons are known
to be toxic. The pigment in the envelope (adventitia) obviate scattering into
the cavity of the head of such light as penetrates directly through the transparent
cornea. We need to remember that the transparent cuticle, namely the cornea,
does not comprise a protective factor here and it is the pigment granules which
assume this role. Note, also, that pigment within the retinular cells can compress
and expand upon exposure to light and can thus act as a sort of shutter (Carlson
et al., 1984). The ocellus is composed of numerous functional units, each of
which is comprised of one comeogenic cell and two retinular cells, each contributing
one rhabdom and creating between them one elongated closed rhabdom in the lower
part (see Figure 2). The corneogenic cell possesses a long process which interpenetrates
between the two retinular cells to reach the rhabdom. Externally, the corneogenic
cells comes into contact with the corneal lens (= cuticle). The region forming
the rhabdom is the distal portion = outer segment of the monopolar neuron, while
the perikarion and the axon comprise the inner segment of the monopolar neuron,
with the latter, in fact comprising a synonym of the retinular cell. Between
the proximal and distal parts of the retinular cell there is a neck of the cell,
traversed by the base of the cilium, which supports the entire outer segment.
Taken as a whole, the corneogenic cell, with the two retinular cells around
its extension and the rhabdom and the inner segments of the two cells, is a
single entity, well separated and delineated. This structure is thus analogous
to the unit of vision in the compound eye, namely the ommatidium, but of a simpler
construction. We, therefore, propose to call this structure by the name ocellon
(which would then parallel the ommatitium).
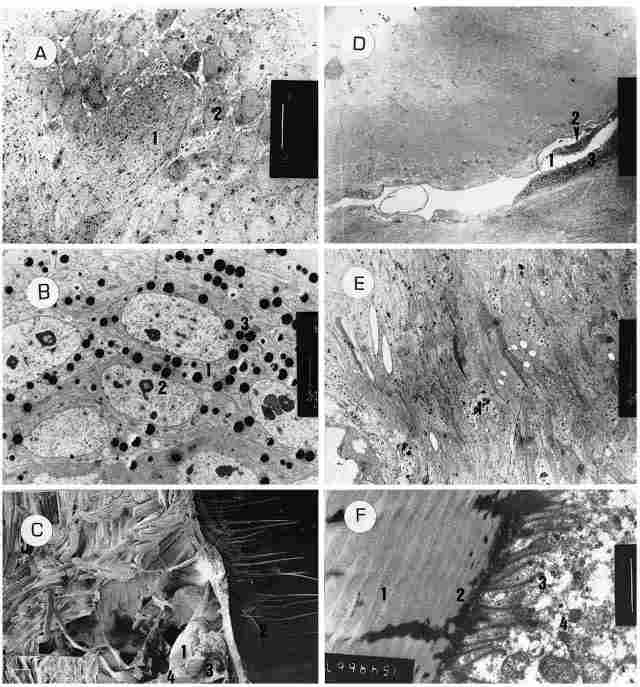
PLATE X: Figure A: On left one can see the axons of the ocellar nerve (1), with
minute pigment granules within the nerve. On right, one can see the bodies of
the monopolar cells from which emerges the axon. Each cell has a large nucleus
(2) and a relatively thin layer of cytoplasm. Bar = 10 Ám. Figure B: Enlargement
of a portion of Figure A. One sees the perikarion of the monopolar cells (I
). Within the cells there are intranuclear inclusions (2). The cytoplasm boasts
numerous pigment granules (3). Bar= 2 Ám. Figure C: A sagittal paramedial SEM
section through the head of a homet. One can see the median ocellus (1) with
the comea (2) on its exterior, while in the inner part can be seen the envelopes
of the ocellus (3) and the ocellar nerve (4). Bar = 100 Ám. Figure D: A view
of the periocellar sulcus (1) which penetrates between the outer layer (2) and
the inner one (3) of the ocellar envelope. Bar = 50 Ám. Figure E. A view of
the corneogenic cells, with their irregularly shaped nucleus (1) and the blackcolored
chromatin granules within the nucleus. Figure F. Section of the cuticle in the
region of the frons (1). One can see the cuticular lamellae, and in the border
between the cuticle and the tissue-an electron-dense layer (2) which is infiltrated
by cilia (3) that originate from the basal body within the cell (4). Within
each cilium one can discriminate between a transparent envelope and a dark inner
region which probably contains microtubules.
The function of the corneogenic cell is to create the cornea above it, to retain
the clarity of that cornea and also physically to support it. The corneogenic
cell constitutes a medium for the passage of light and is consequently perfectly
clear, without any pigment granules. The process of the corneogenic cell transports
impinging light down to the rhabdom, which is located in the proximal third
of the outer segment, that is, in the deep part of the ocellus. It can thus
justifiably be compared to an optic fiber. In many respects, the corneogenic
cell resembles functionally the vitreous body in the eyes of vertebrates. An
ancillary, but no less important role of the corneogenic cell is to support
the retinular cells and keep them apart, otherwise if they coalesce, no light
can enter and pass. As mentioned, the corneogenic cell is totally devoid of
melanin, which leaves unclarified the manner whereby free radicals formed in
the course of light passage are neutralized.
The Retinular Cells and the Rhabdom
The role of the rhabdom is to pick up light stimuli and convert them
into a bioelectric potential. This is achieved via retinoids located in the
folds between the membranes. The energy assumes the form of ATP and the retinoids
insinuate into the folds of the rhabdom through the microtubules present in
the folds. After the exposure to light, remnants of the "used" membrane are
ejected into the cytoplasm. The function of the microtubule is to provide metabolic
supply and structural support to the rhabdom which must remain straight, because
any bend or twist of the rhabdom is liable to exclude parts of it from the pathway
of the light rays that pass through the process of the cornengenic cell and
thereby diminish its efficiency. The rhabdorn apparently is composed of the
plasmalemma of the two retinular cells, with the contact between the two membranes
being very tight and in fact resulting in a fusion having the nature of a full
desmosome between the two membranes. Structural elements like microtubules and
microfilaments intercross from one cell to the other, thus ensuring the stability
of the rhabdom without any relative movement between the membranes from the
two cells. This also guarantees complete synchronization, so that the cells
undergo depolarization concurrently and thus function as a single unit. All
this leads to the conclusion that there is also functional justification for
calling the separate units by the name ocellon. In several of the pictures,
on crosssection, the rhabdom appears as if bent at the base region. This, however,
is not a bend in the rhabdom, however rather the figures of an oblique section.
The process of light uptake by the rhabdom and its conversion to a bioelectric
potential requires a large amount of energy which is supplied by the very large
number of mitochondria. The mitochondria are densely concentrated around the
rhabdom itself and to a much lesser extent in other parts of the cell. In this
connection, the described structure is similar to that ascribed for the ommatidia
(Carlson et al., 1984). The perikarion is relatively slender and contains a
nucleus with a thin layer of cytoplasm around it. Glia cells are distributed
among the perikarion cells. The contact between the glia cells and the perikarion
cells is attained via septate junctions that enable passage of intracellular
metabolites from cell to cell. These glia cells are analogous to the Samper
cells (Saint Marie et al., 1984) which support the neurons of the ommatidia.
The necks of the monopolar cells in the base of the outer segments are embedded
within a special cuticular layer of an areolar structure. The ocellus tends
to accumulate heat owing to the rapid metabolic process as well as to solar
irradiation which is concentrated in the ocellus by the transparent cornea (which
acts as a concentrating lens) and therefore flow of hemolymph is important for
cooling of the ocellus. The areolar region is located as a continuation of the
periocellar sulcus which likewise enables flow of hemolymph to and fro.
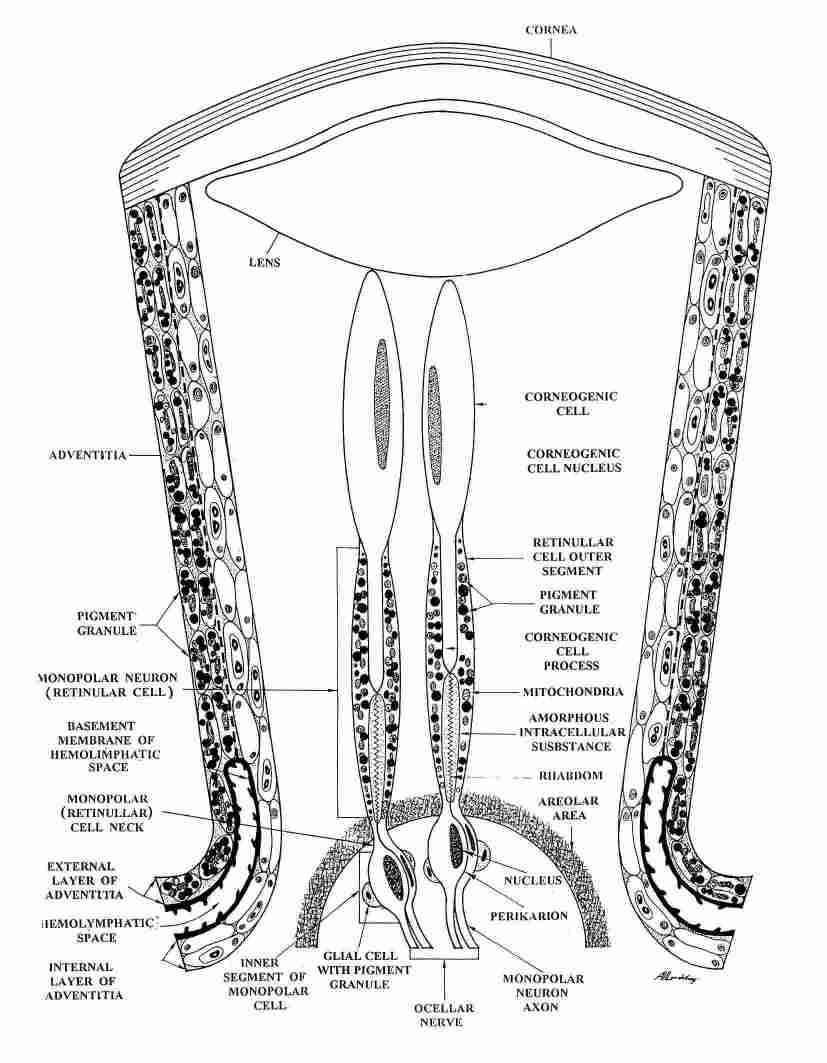
FIGURE 2. Schematic representation of the units in the ocellus. Each unit is
comprised of one comeogenic cell and two retinular cells, each contributing
one rhabdomer and creating between them one elongated closed rhabdom in the
lower part. Note the long process of the comeogenic cell reaching the rhabdom.
For more details see text.
Arrangement of the Ocelli in the Hornet's Head
The ocelli are situated on the vertex plate in such fashion that if we draw
a straight line through each, we end up with an equilateral triangle. Each ocellus
bears a convex cornea shaped as a hemisphere. The tangential planes of each
ocellus create between them a pyramid of three equal sides. The arrangement
of each ocellus on a different plane enables a panoramic visual coverage of
practically all the 360 0 field of vision above and around the head. On our
stated assumption that the ocelli pick up polarized light we surmise that hornets
can sense the direction of the sun rays through them. It is customary in the
Vespan nest that only the adult hornets depart the nest to forage in the field
for food or other nest necessities, whereas the young hornets remain in the
nest up to an age of 3-5 days after eclosing. Thereafter, and during their initial
forays out of the nest, the now adolescent hornets take wing only for brief
time intervals, flying in ever wider circles around the nest entrance (Gaul,
195 1; Spradbery, 1973; Ishay and Rosenzweig, unpublished observations). During
these initial flights the hornets apparently acquaint themselves with the site
of the nest and its immediate environment, as well as with its situation in
relation to the fixed angle of the sun to the zenith. When the hornet departs
the nestsomething it does only during the day time it gauges the direction of
the sun relative to the zenith while performing circular flying movements around
the nest entrance and then as it turns in the direction of the sun, its median
ocellus becomes equally illuminated throughout, while the lateral ocelli are
lit only in the parts facing the sun, leaving other parts relatively shaded.
When the sun is at an acute angle, the upper parts of the body and the ocelli
become illuminated, whereas when the angle is obtuse, it is the lower parts
which are insulated. Thus by relying on the angle of the sun, a hornet can locate
and assess the intensity of the transverse axis in respect to north or south
and similarly also the directions of east and west. In other words, by employing
circular flying movements upon departing the nest or returning to it, the hornet
can orient itself in accordance with the direction of the sun rays impinging
upon its ocelli. Inasmuch as the location of the nest in respect to east-west
north-south is invariably fixed, the hornet learns to find it, that is, undergoes
imprinting on it, and thereafter is capable of returning to the nest by a direct
route from any point in the field without having to retrace its flight pattern
from the nest to the specific point in the field from which it is returning.
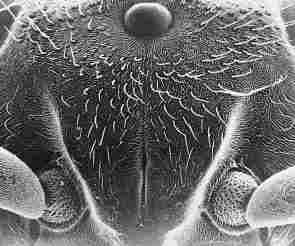
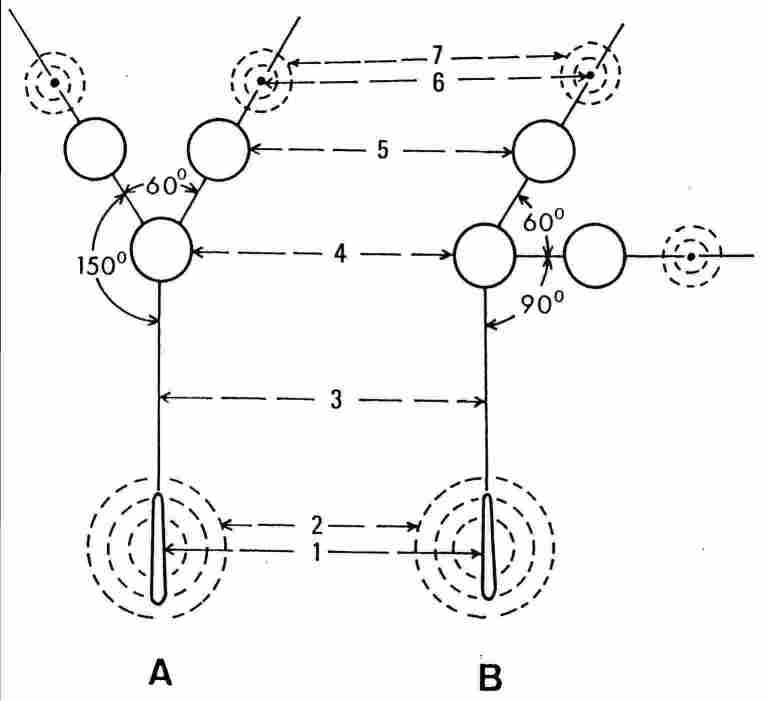
FIGURE 3. SEM view of the frons and adjacent area of a homer worker Vespa orientalis
and a schematic presentation of the three ocelli and their respective glands
(the paraocellar organ).
A: An over-simplifed schematic view of the ocelli and glands shown on the same
plane. B A schematic view showing the real position of the ocelli and paired
statocyst glands on the vertex, and the median statocyst gland on the firons
plate. 1, sutura coronalis; 2, 7, semicircular or circular rows of seme around
the sutura coronalis and around the glands adjacent to the lateral ocelli; 3,
the depression on the frons between the median ocellus and the Coronal suture
4, the median ocellus; 5, one of the lateral ocelli; 6. (analogous to 1), a
gland adjacent to one of the lateral ocelfi. For more details see text.
The ocelli enable the hornet to determine the direction of the sun. However,
to be able to determine the direction of the sun vis-a-vis the zenith, the hornet
needs to orient itself in a position which is absolutely horizontal with respect
to the earth's surface or, alternatively, with respect to a hypothetical plane
which is tangential to the earth's surface. For this purpose, the hornet relies
on two organs of equilibrium located at some remove from the two lateral ocelli,
and a third, within the coronal suture which extends from the median ocellus
(Figure 3). With the aid of its equilibrium organs, the hornet locates the zenith,
while placing itself in flight in a perfectly horizontal position so that it
can measure the angle of the sun in respect to the zenith (Ishay and Ganor,
1992). By way of analogy, we could say that the hornet uses its organs of equilibrium
to calibrate its 'measuring device' to the zenith. Regarding the question as
to why each ocellus needs its own equilibrium organ, we speculate that this
enables each ocellus to obtain information pertaining to its position vis-a-vis
the direction of the gravitational pull. This is in fact a complex organ containing
both organelles for sensing gravitation as well as ones for sensing light and
there are undoubtedly also elements that integrate between the two types. We
presume that such integration takes place within the protocerebrum. The organ
is a type of accelerometer, that gauges the direction of light in respect to
the direction of gravitation. Interestingly, we note that even when one anesthetizes
the nest or seals its entrance the foraging hornets returning from the field
and failing to find the nest entrance or enter it, soar upward again to fly
in circles around the nest entrance, apparently for purposes of reorientation
(Ishay and Rosenzweig, unpublished observations). The structure described herein
comprises an ocellon provided with a closed rhabdom that is located in depth
and is connected with a long lens, i. e., the long corneogenic cell process.
Such a structure allows penetration of polarized light of short wavelength only.
This light of short wavelength needs to penetrate deeply because we are dealing
with the configuration of a scotopic eye (Gillot, 1995). As pointed out, the
structure is geared for penetration of polarized light because only the segment
of light which travels vertically in the 'canal' (i. e., the relatively long
extension of the corneogenic cell) is capable of reaching the rhabdom. Bear
in mind that the rhabdom is straight and the light has to pass through its entire
length. It has been reported that in the bumblebee the strongest response in
the ocelli was to UV light while the response to green light was weak, which
is contrary to the situation in the compound eye (Menzel and Snyder, 1974; Menzel
and Blakers, 1976). Similar findings have been reported in the Xiphosuran Limulus
(Chapman and Lall, 1967) and in Calliphora fly (Kirshfeld and Lutz, 1977). As
for the honeybee, it seems fairly clear that the cells sensitive to UV are the
ones that are responsible for picking up polarized light (van Helversen and
Edrich, 1974). According to Wellington (1974), the bumblebee's ocelli are sensitive
to polarized light and they are the ones which are responsible for twilight
navigation. According to Frisch (1967), the honeybee likewise navigates by relying
on polarized light. Conceivably, then, in the Oriental hornet the ocelli contribute
both to orientation and navigation thanks to their sensitivity to polarized
light. The morphology of the ocelli, as afore described, certainly justifies
concluding that they have some role in hornet vision. Indeed, the hornet possesses
an array of visual structures, towit: compound eyes, ocelli and also very numerous
peripheral photoreceptors which are distributed over the entire body at varying
densities in different regions. We are still in the dark, mostly, regarding
the disparate roles of the various organs intended for reception of light; what
is certain is that hornets do not fly in the dark, that is, they do not depart
the nest at night. When we cover their compound eyes (ommatidia) with paint
(Tippex), thus artificially creating a state of darkness (of their compound
eyes), the 'painted' hornets attempt to fly since, we presume, the other light
receptive organs are still exposed to optic stimuli. Yet these hornets are incapable
of proper navigation and lack the certainty of reaching their target outside
the nest and therefore they do not persist in flying and none of them is able
to return to the nest. In comparing hornets whose ocelli were 'covered' and
ones whose ommatidia were 'covered' we find that some of the former succeed
in returning to the nest. This means that masking of the ocelli does affect
the navigatory capability of the hornets. At least some of the hornets (about
35%) are still able to navigate their way home. Another parameter affected by
the diminution of navigation is the time interval required from release of the
treated hornets outside the breeding box and their successful return. Thus,
the ocelli masked hornets require more time to return than do the control nonmasked
hornets and on occasion some of the test hornets return only on the next day.
The exact role of the peripheral receptors is still unclear, but we do know
that they, too, are sensitive to light and play some role. Admittedly, the present
preliminary findings have not unraveled the precise role of the ocelli in vespan
navigation, clearly they comprise an important and essential component in the
navigatory powers of the hornet.
References
-Baumann. F. (1975) Electrophysiological properties of the honey bee
retina. In: The Com pound Exe and Vision of Insects,( G. A. Horridge, ed), Oxford
University Press (Clarendon) London, pp. 53-74.
-Carlson, S. D., Saint Marie, R. L. and Chi, C. (1984) The Photoreceptor Cells.
In: Insect Ultrastrucane. (R. C. King and H. Akai, eds), Plenum, New York, 2(
Il): 397-433.
-Chapman, R. M. and Lall, A. B. (1967) Electroretinogram characteristics and
the spectral mechanism of the median ocellus and lateral eye in Limulus. J.
Gen. Physiol. 50: 2267-2287.
-DukeElder, S. Sir. (1958) The eye in evolution. In: System of Ophthalmology,
(S. DukeElder, Sir, ed), London: Henry Kimpton, pp. 152-225.
-Edrich, W., Neumeyer, C. and Helversen, O. V. (1979) "Antisun" orientation
of bees to a field of ultraviolet light. J. Comp. Phys. 134: 151-157.
-Frisch, K. V. (1967) The Ounce Language and Orientation of Bees. Belknap Press
of Har vard Unkersity Press, Cambridge, Mass.
-Gaul, AT. (1951) Additions to vespine biology. VIL Orientation flight. Bull.
Brooklvn Ent. Soc. 46: 54-56.
-Gillet, C. (1995) EntoinologvCompound Eves, Plenum Press, New York 2nd ed..
12: 378-389.
-Goldsmith. T. H. and Ruck, P. (1958) The spectral sensitivities of the dorsal
ocelli of cock roaches and honeybeesan electrophysiological study, J. Gen. PhYsiol.
41: 1171-1185.
-Helversen, O. V. and Edrich, W. (1974) Der Polarisationsemphanger in Bienenauge:
ein Ultraviolet Receptor. J. Comp. Phvs. 94: 33-47.
-Hesse, R. (1907) Das Sehen der mederen Tiere, Fischer, Jena.
-Imms, A. D. (1960) A General Textbook of Entomology, 9th ed. London, Methuen
& Co. Ltd., 84114.
-Ishay, J. (1964) Observations sur la biologie de [a Gu6pe orientate Vespa orientalis
en lsraeI. Insectes Sot iaux XL 193206.
-Ishay, J. S. (1975) Caste determination by social wasps: Cell size and building
behaviour. Amm. Behat. 23: 425-431.
-Ishay, J. S. and Ganor, E. (1992) External micromorphology of the frons plate
and its adja cent areas in workers of the Oriental hornet. J. Morph. 213( 1):
113.
-Jongebloed, W. L., Dunnebier. E. A.. Albers, F. W. J. and Kalicharan, D. (1996)
Demonstra tion of the stereocilia fine structure of the organ of Corti of the
guinea pig by field emis sion scanning electron microscopy (FEGSEM). Scan. Microsc.
10( l): 147--164.
-Jongebloed. W. L., Rosenzweig, E., Kalicharan, D., Want, v. d. J. J. L. and
Ishay, J. S. (1999) Ciliary hair cells and cuticular photoreceptor of the hornet
vespa orien talis as components of a gravity detecting system: an SEM/ TEM investigation.
J. Elec tron. Microsc. 48( l): 63-75.
-Kirschfeld. K. and Lutz, B. (1977) The spectral sensitivity of the ocelli of
Calliphora (Di tera). Z fur Naturforschung 32( C): 439-441.
-Locke. M. (1984) The structure and development of the vacuolar system in the
fat body of insects. In: Insect Ultrastructure, (R. C. King and H. Akai, eds),
Plenum, New York, 2( 5): 151-197. Menzel, R. and Snyder, AW. (1974) Polarized
light detection in the bee Apis mellifera. J. Conip. Phvs. 88: 247-270.
-Menzel. R. and Blakers, M. (1976) Colour receptors in the bee eyemorphology
and spec tral sensitivity. J. Comp. Phvsiol. 108: 11-33.
-Muller, E. (1931) Experimentelle Untersuchnung and Bienen und Ameisen. Cher
die Funktions" eisen der Stimocellen. Z. vergl. Phisiol. 14: 348-384.
-Richards, OW. and Richards, M. J. (1951) Observations on the social wasps of
South America (Hymenoptera, Vespidae). Trans. R. ent. Soc. Land. 102: 11-70.
-Saint Marie, R. L., Carlson, S. D. and Chi, C. (1984) The glial cells of insects.
In: Insect Ultrastructure, (R. C. King and H. Akai, eds), Plenum, New York,
2( 12): 435-475.
-Snodgrass, R. E. (1935) Principles of Insect Morphology. New York, McGrawHill
Book Co., Inc.
-Spradbery, J. P. (1973) Wasps. London: Sidgwich & Jackson. Stusek, P. and
Gogala, G. (1971) Spectral sensitivity of the ocellus and the compound eye of
the bug Oncopeltusfasciatus. Biolski Vestnik, 19: 103-108.
-Tracopoulos, M., Poitry, A. and Borsellina, A. (1981) Diffusion and consumption
of oxygen in the superfused retina of the drone (Apis millifera) in darkness
J. Gen. PhIsiol. 77: 601-628.
-Wehner, R., Bernard, G. D. and Geiger, E. (1975) Twisted and nontwisted rhabdoms
and their significance for polarization detection in the bee. J. Comp. Physiol.
104: 225-245.
-Wellington, W. G. (1974) Bumblebee ocelli and navigation at dusk. Science 183:
550-55 1.
-Wigglesworth, V. B. (1965) The Principles of Insect Physiology. Methuen, London.