Chapter 7
Submitted to Journal of Vestibular Research
Abstract
We studied vestibular function in 20 adult
hamsters (3 months old) subjected to either prolonged hypergravity (n=10) or
normal gravity (n=10) for 2 months. The motor coordination of the hypergravity
(HG) hamsters hardly changed; locomotion and swimming under light conditions
was normal. Equilibrium maintenance was severely disturbed; only 6 of 10 hypergravity
hamsters managed to walk on the small tube after 2 months, whereas all 10 controls
(CON) were able to walk on the tube. Hypergravity hamsters were also less susceptible
to rotations and the air-righting reflex was severely disturbed; the HG hamsters
made 30% correct air-righting responses, while the CON hamsters made 88% correct
responses Finally, 5 of 8 HG hamsters had to be saved from drowning when swimming
in total darkness. Histological examination of the utricular otoconial layers
afterwards, using energy dispersive X-ray element analysis and scanning electron
microscopy, showed no changes in calcium content, size, shape and size distribution
of the otoconia.
We conclude that adult hamsters adapt to hypergravity, which leads to problems
in normal functioning when tested under normal gravity conditions, especially
in tasks in which sensory input of the vestibular system is the dominant source
for orientation. These disturbances are more severe in adult hamsters than in
young ones tested in previous experiments, so it is assumed that age is a factor
for adaptation to altered gravity conditions.
Keywords: centrifugation, balancing, air-righting reflex, swimming behaviour, otoconia.
Introduction
Exposure to altered gravity can lead to
a syndrome of motion sickness. Space research has shown that 40% to 60% of all
astronauts experience a kind of motion sickness (Space Adaptation Syndrome)
during the first days in space (Thornton et al. 1987). Furthermore, astronauts
subjected to weightlessness for 8 to 10 weeks, show balance disturbances on
tests after return to normal gravity. These disturbances last for several days
(Homick et al. 1977; Young et al. 1984). After an increase of gravity (hypergravity
or HG) in a centrifuge, astronauts experience the same motion sickness symptoms
in normal gravity as the ones they had experienced after their spaceflight (Bles
et al. 1989). Postural instability and "stiff robot-like behaviour" during walking
in normal gravity was also found in several subjects exposed to hypergravity
(3 G) for 1.5 hour (Bles and de Graaf, 1993). These data indicate an involvement
and possibly an adaptation of vestibular system to weightlessness or hypergravity,
which is probably the same mechanism for both conditions. Therefore, hypergravity
experiments in a centrifuge could facilitate research to this adaptation mechanism.
In earlier experiments we developed an animal model for studying the effect
of hypergravity on vestibular induced behaviour. Although signs of motion sickness
were missing, we found balance disturbances during normal gravity in young hamsters
subjected to hypergravity (2.5 G) in a centrifuge for 4 or 6 months (Sondag
et al. 1995a; Sondag et al. 1996). These disturbances were observed during the
first weeks of exposure and disappeared after several weeks probably because
of the training experience on the tubes. However, one has to take into account
that these hamsters were only 3 weeks old when exposed to hypergravity. For
comparing the results of human and animal experiments in hypergravity it is
necesarry to compare adults.
Therefore, the present study was performed in which we investigated the behaviour
of adult hamsters after longterm exposure to hypergravity. Attention was paid
to their locomotion pattern during normal walking, balance tests and during
exposure to rotatory accelerations. Other tests, capable of detecting differences
in vestibular functioning, such as air-righting and swimming (Lim and Erway,
1974; Petrosini, 1984; Huygen et al. 1986; Pellis et al. 1989, Llorens et al.
1993), were also applied. Afterwards, the utricular otoconial layer was histologically
examined to assess the effect of hypergravity on the otoconial morphology.
Material and methods
Centrifuge: The centrifuge consisted of a centrally placed 3.5-kW DC motor drive and 2 horizontally mounted arms (length = 115 cm) with aerated and darkened free-swinging gondolas (length: 110 cm, width: 45 cm, height: 80 cm, length arm + gondola: 194 cm). At a rotation speed of 34,3 rpm a 2.5 gravity-value was reached at the bottom of the gondola.
Animals: The study was done in a
group of 20 male adult golden hamsters (Mesocricetus auratus). The hamsters
were born and raised by Harlan (Zeist, The Netherlands) and were 3 months old
with a mean weight of 90 g at the start of the experiment. Ten of them were
placed in acrylate boxes (22 cm x 37 cm; 3 or 4 animals per box), inside the
centrifuge-gondola to live under conditions of 2.5 G (HG hamsters). A video
camera in the gondola made it possible to observe the animals' behaviour during
hypergravity. The other 10 hamsters (CON hamsters) were placed in similar housings
under normal gravity. Food and water were available ad libitum and the
day-night cycle (light on 19.00 - 7.00 h) was reversed. During the centrifuge
stops (30 minutes), the hamsters were tested in a laboratory room with dimmed
lights. Additional noise (radio) was present to avoid distraction of the hamsters
by the experimenter. The body weight was measured twice a week.
After two months, the hamsters were killed for histological and morphological
examination of the otoconia of the saccule and utricle. The experiments were
performed in accordance with the principles of laboratory animal care and with
the recommendations provided in a special licence as required by the Dutch Law
on the Use of Animals in Scientific Research.
We registered the stride, tube and swimming tests weekly and the rotation and
the no-rotation task monthly. The air-righting reflex, resurfacing and swimming
in the dark were tested in the last week of the experiment (week 8).
Tasks
Stride task: The hamsters were held by their
chest and their front and hind paws dipped in a tray containing film developer
(Agfa) and had to walk in a small plexiglass walkway (length 43 cm, width 9.5
cm) in which an undeveloped X-ray film (Curix) was laid. The film with the paw
prints were dipped in rapid fix (Agfa) for 5 minutes, washed for 10 minutes
and dried (method earlier described by de Medinaceli et al. 1982). For evaluation
of the strides we used the method described by Hruska et al (1979). We measured
the distance between consecutive left hind pawprints (step-length), the distance
of the interposed right hindpaw perpendicular to the line connecting the two
consecutive left hindpaws (step-width) and the distance between the ipsilateral
fore and hind paw prints (superimposed step). At the same time, the dynamic
aspects of the walking behaviour were stored on tape. The videorecorder (Panasonic,
NV-FS90) was able to show intervals of 20 ms (50 tracks per second). We counted
swing time (elevation and forward movement of the foot) and stance time (placement
of the foot upon the floor until the next swing) and calculated the percentage
of swing time (swing time / swing time + stance time).
Tube task: the acrylate tube (length 100 cm, diameter 20 mm), placed ± 20 cm
above ground level, was fixed to standards and covered with tape (Leukosilk,
Beiersdorf AG, Hamburg, Germany) in order to give the hamster a better grip.
A platform was placed at the end of the tube where the hamster could collect
sun-flower seeds for ± 5 s. One extra day was used to train the hamsters for
5 minutes to walk the full length of the tube. Each hamster had to cross the
tube 3 times. After 7 days of HG, the hamsters were tested on the tube once
a week. We measured the time to cross the tube (crossing time) and fall frequency
per trial.
Rotation task: A hamster was placed in a cylindrical bucket (diameter 30 cm,
height 45 cm) mounted on a swivel tool device with 10 sinusoidal pendular rotations
at a frequency of 0.1 Hz and an amplitude of 900 (total swing time
100 s). The number of changes in walking direction (turnings) was counted in
this task and also for 100 s when the device did not move (No-rotation task).
The period between the rotation and the no-rotation task was 2 weeks.
Swimming in a lane (140 x 10 cm, height of the walls 30 cm, water-depth 25 cm,
water temperature ± 300 C) to test swimming ability and speed. The
hamsters had to swim to the end of the lane where they could climb an escape
ladder. One extra day was used to train the hamsters for 5 minutes to swim to
the ladder. On the testing days, the animals swam 6 trials; 3 in the lane and
3 in the maze. The crossing time for the middle part of the lane (length 100
cm) was measured.
Orientation ability during swimming in a maze (140 x 70 cm). The shape of the
maze was similar to our earlier experiments (Sondag et al. 1995a; Sondag et
al. 1996a). The hamsters had to find a ladder, located at the opposite end of
the maze. The crossing time was measured and the orientation strategy (swimming
along the walls or straight through the center of the basin) was observed.
Air-righting reflex, resurfacing and swimming
in infrared light conditions
In week 8, to assess the hamsters air righting reflex, 8 HG hamsters and 8 CON
hamsters were dropped in a supine position from a height of 80 cm into a water
basin. After resurfacing the hamsters had to swim for 30 sec. To avoid visual
input this test was performed under infrared light conditions (870 nm) in which
the hamsters are unable to use visual information. The hamsters behaviour was
recorded on video for later analysis. Three succesive trials were given. We
measured the number of correct air righting responses (landing with feet parallel
to the surface of the water), the time needed to resurface after hitting the
water and the swimming ability in the dark during 30 s.
Histology
The HG and CON hamsters were killed and the temporal bones were dissected. The utricular patches were fixed in 2.5% gluteraldehyde + 0.5% paraformaldehyde in phosphate buffer solution (0.1 M, pH 7.4). After rinsing in distilled water and air-drying, the specimens were prepared for calcium content analysis and scanning electron microscopy.
To determine the calcium content of the otoconia, the specimen were sputter-coated with carbon and subjected to energy dispersive X-ray (EDAX, DX4) element analysis. The data were analysed with the help of Phizaf (EDAX, Mahwah, USA). For electron microscopical scanning (ISI SS40), the patches were mounted on aluminium stubs and coated with gold. Photos were made to determine the effect of hypergravity on size and shape of the otoconia and on the areas with small, medium-sized or larger otoconia. Measurement of the otoconial distribution on the utricular patch was done by three different experimenters and the method was the same as described in Sondag et al. (1995b). There was no inter-observer difference in measurement of the areas. Therefore, the data of one investigator (HNPMS) are presented.
Data were statistically assessed (significance: p<0.05) with repeated analysis of variance (weight, gait, tube task, swimming speed, susceptibility for orientations), one-way ANOVA (resurfacing, swimming in the dark, otoconia distribution) and the binomial test (air-righting reflex). We used the statistical software SPSS PC+ 5.0 for our analysis.
Results
The body weight increased in all 20 hamsters, no significant difference in body weight gain was found between HG hamsters and CON hamsters.
Stride test: No differences were found in length and width of the steps between HG and CON hamsters. Both groups increased the step-length (F(6,108)=7.51, p<0.001) and the step-width (F(6,108)=6.15, p<0.001) during the experiment. Concerning the superimposed step, we found that the distance between the ipsilateral front and hind leg was larger for HG hamsters than for CON hamsters (F(1,18)=7.15, p=0.016). No differences between HG and CON hamsters were found in the dynamic aspects of walking; walking speed (varying between 12 - 40 cm/s), swing time, stance time and percent of swing time (swing time / swing time + stance time) was the same for both groups.
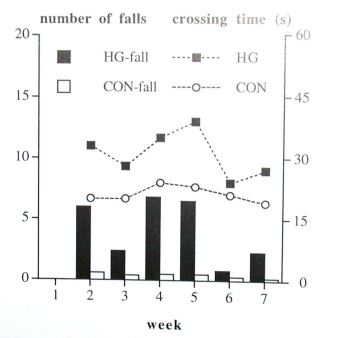
Equilibrium maintenance on the tube: All 10 control hamsters were able to walk on the tube after the pretraining day and the first testing day, whereas 3 of the 10 HG hamsters were able to walk on the tube. After 8 weeks 6 of the 10 HG hamsters and all 10 CON hamsters were able to walk on the tube. The HG hamsters who walked on the tube after 8 weeks needed more time to cross the tube (mean HG hamsters 27 s, mean CON hamsters 19 s, not significant) and fell down more often than control hamsters (mean HG hamsters 2.30 times, mean CON hamsters 0.20, F(1,10)=13.74, p=0.004; fig. 1).

Fig. 1. Number of falls and crossing time on the small tube. Bars represent the number of falls (Y1-axis), lines represent the crossing time (Y2-axis). Shown are means.
Susceptibility to rotatory accelerations: During the rotation task the HG hamsters made less turnings than the CON hamsters (F(1,18)=7.86, p=0.012). Both groups increased the number of turnings in the rotation task during the experiment (F(2,36)=6.17, p=0.005). In the no-rotation task, no differences were found in the number of turnings between both groups (fig. 2).
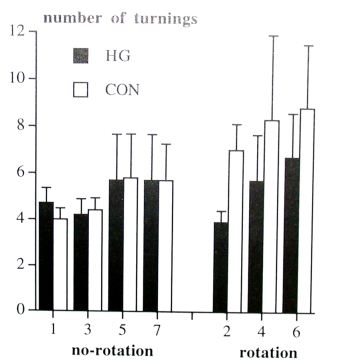
Fig. 2. Number of turnings during the no-rotation task (week 1, 3, 5 and 7) and during the rotation task (week 2, 4 and 6). Shown are means and standard deviations.
Swimming in the lane: No differences were found in the swimming ability and speed in the lane between HG and CON hamsters, both groups increased their swimming speed during the experiment (F(4,64)=4.65, p=0.003).
Swimming in the maze: No differences between the two groups were found in time needed to find the stair and the orientation strategy.
Air-righting reflex, resurface time and swimming in infrared light conditions: Over all trials, we observed correct air-righting reflexes in 30% of the HG hamsters and in 88% of the CON hamsters (p<0.000). Time needed to resurface from the water was 1.05 s for the HG hamsters and 0.67 s for the CON hamsters (p=0.024). Concerning swimming in infrared light, 5 of 8 HG hamsters had to be saved from drowning, whereas all 8 CON hamsters swam normally.
Element analysis
For EDAX element analysis utricular patches were used from 3 HG hamsters and
3 control hamsters. We found that the calcium content was the same for otoconia
with different sizes, no differences were detected in calcium content between
large otoconia and small otoconia. With regard to differences in calcium content
between HG animals and controls, element analysis revealed no differences between
the otoconia of the HG hamsters and the otoconia of the control hamsters. Calcium
peaks were detected for both groups, and no difference was observed in the height
of the peaks.
Otoconial distribution on the utricular
patch
Concerning the size of the otoconia small, medium-sized and large otoconia were
found in the utricle of both HG hamsters and controls. Furthermore, we found
that the otoconial distribution of the macula utricle was the same in HG hamsters
and control hamsters.
Discussion
Several authors reported that longterm exposure
to altered gravity conditions induced a functional adaptation in visual, vestibular
and somatosensory systems leading to motion sickness, balance disturbances and
locomotion disorders (Reschke et al. 1986; Young et al. 1986; Bles et al. 1989;
Paloski et al. 1993; Bles and de Graaf, 1993, Dai et al. 1994). It is hypothesized
that after longterm exposure to microgravity or hypergravity, the altered signals
from the adapted otolith organs results in postural disturbances directly after
return to normal gravity (Paloski et al. 1993).
In the present experiment, we studied the behaviour of adult hamsters exposed
to hypergravity for two months. During the first week of HG exposure the weight
of the HG hamsters decreased (not significantly) but after several days it increased
to the values of the CON hamsters. This increment in body weight to control
level in the present experiment was not observed in studies with young hamsters
exposed to hypergravity after weaning (Briney and Wunder, 1962; Sondag et al.
1996) or in young and adult rats (Oyama and Platt, 1964; Pitts et al. 1972).
The cause of this increment in adult hamsters remains unclear. The body weight
decrease during the first days of HG exposure was found in all experiments and
is explained by the reduced food and water consumption during these days which
is assumed to be caused by stress effects (Briney and Wunder, 1962; Pitss et
al. 1972; Economos et al, 1982).
The HG hamsters showed normal gait and stride during walking in 1 G. The only
significant difference we found was that the distance between the hind paw prints
and the fore paw prints (superimposed step) was larger in HG hamsters then in
CON hamster but this did not affect gait. Balancing on the small tube, however,
was severely disturbed; 4 out of 10 HG hamsters had difficulties with standing
on the tube and were incapable to walk on the small tube even after 8 weeks
of testing. In the tube task the animals were forced to keep balance during
locomotion while step-width was held small (± 3.7 cm during normal locomotion
versus 2 cm when walking on the tube). This decreased step-width combined with
the disturbances in locomotion, as were found in the stride task, could have
resulted in a decreased balance on the tube, because the proper corrections
could not be made. In earlier experiments, we found that young HG hamsters had
less difficulties with learning this task and after several weeks performed
just as well as control hamsters (Sondag et al, 1995a; Sondag et al, 1996).
In addition, the adult CON hamsters performed just as well as young ones of
previous experiments thus excluding the possibility that a higher center of
gravity caused these disturbances in the adult HG group. We hypothesize that
this difference in balancing between young and adult HG hamsters is an age effect.
For sensory motor control of posture and balance, integration of visual, somatosensory
and vestibular information is required. During early childhood the value of
the input coming from these systems is weighted. Exposure to hypergravity during
childhood changes the integration of these inputs. Therefore, the young animal
seems to be better capable to functionally adapt to the alterations in G-load
and switch to sensory information sources other than vestibular.
The swimming ability of the adult HG hamsters and their age-matching controls
was only disturbed during swimming in the dark. When visual information was
available, the HG hamsters swam just as fast and used the same orientation strategy
as the CON hamsters and as young CON and HG hamsters during the first testing
months (Sondag et al. 1996). Furthermore, both the decreased performance of
the air-rightting reflex (absence of visual and somatosensory information) and
the decreased susceptibility to rotatory accelerations (absence of adequate
visual cues) suggest that longterm exposure to hypergravity results in a decreased
performance on tasks in which subjects have to rely on input from the peripheral
vestibular system. Our data support the hypothesis made by Paloski et al. (1993)
that a change in otolith information causes these disturbances.
As far as the morphology of the vestibular end organs is concerned, we found
no alterations in calcium content, size, shape and distribution of the otoconia
on the utricular layer in adult HG hamsters, which is the same outcome as we
have found for the young HG hamsters (Sondag et al. 1995b). The conclusion of
Sondag et al. (1995b) that otoconia of animals exposed to hypergravity after
birth show no structural adaptation is thus confirmed by the present study.
Differences in the otoconia distribution were observed in hamsters conceived
and born under hypergravity conditions (unpublished data). This shows that during
the embryonal period the vestibular end-organs are capable of adapting to an
altered gravity. Adaptation in other parts of the otolith organs, such as the
hairbundles or haircells, or in the integration and storage of information in
the central part of the vestibular system could be the underlying cause of the
behavioural disturbances found in hamsters exposed to hypergravity after birth.
This must be explored further in the future.
We conclude that longterm exposure to hypergravity in adult hamsters resulted
in behavioural disturbances on vestibular-dependent tasks. These disturbances
are more severe in the adult hamsters than in young ones, so it is assumed that
age is a factor for the adaptation mechanism to hypergravity.
Acknowledgements
The authors gratefully acknowledge the Netherlands Organization for Scientific Research (NWO) for funding this project. This research was conducted while HNPM Sondag was supported by a grant of the Foundation for Behavioural and Educational Sciences (SGW) of this organization (575-62-049), awarded to Prof. Dr. WJ Oosterveld.