Chapter 2
Anatomy and function of the vestibular system
The vestibular system is, with the visual and somatosensory
system, involved in the control of balance and orientation. It contains the
peripheral vestibular receptors, located in the inner ear and the central vestibular
nuclei, located in the brainstem.
The peripheral vestibular receptors are within the labyrinth, which is filled
with perilymphatic fluid and suspensory connective tissue used to avoid direct
shocks. The sensory structures in the labyrinth responsible for the transduction
of vestibular information in mammals are the otolith organs and the semicircular
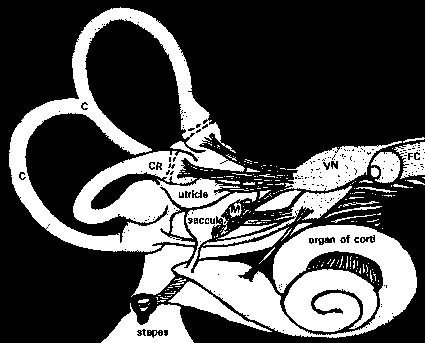
canals. (Fig. 1)

Fig. 1: Semicircular canals and the otolithic organs. C: semicircular canals; CR: crista ampullaris; M: macula; VN: vestibular nerve; FC: facial nerve (modified from Parker, 1980).
The peripheral vestibular system has four major functions: a) maintenance and regulation of the tonus of the body musculature; b) detection of rotatory accelerations; c) detection of linear accelerations and d) detection of vibrations (Lowenstein, 1974). These functions are accomplished by transducing the forces associated with head/body acceleration and alterations in gravity into a biological signal. The signals are transported via several nerve pathways to the "control centers", amongst others, the central vestibular nuclei (Gacek, 1981). These centers process the vestibular signals together with signals arriving from the visual and somatosensory systems. The output from the central vestibular nuclei is used to produce reflexes for equilibrium maintenance and locomotion, and for spatial orientation.
The emphasis in this thesis is on the structure and function of the otholithic organs, e.g. the utricle and saccule. The morphology and physiology of these structures are discussed in detail while the other parts of the vestibular system, the semicircular canals and the central vestibular system, will be discussed briefly.
2.1 Otolith organs
2.1.1. Anatomy
The form of the utricular and saccular maculae can vary considerably between species. In mammals, the utricle is heart-shaped while the saccule is hook-shaped. The plane of the macula utricle is mostly horizontal except the anterior part that is elevated about 300 with reference to the plane of the horizontal canal. The plane of the macula saccule is roughly vertical. The macula can be divided into two areas (the pars interna and the pars externa) by a curved line in the central region of the macula, the striola (fig. 2). The striola is characterized by the presence of dense sensory cells with shorter stereocilia over this region than in the other parts of the macula. In the utricular macula, the otoconial layer is thinnest over the striola region with the large otoconia lying in the lateral part of the macula. In the saccular macula a thicker layer of small otoconia was found in the striola region with a concentration of type I hair cells (Lindeman, 1969).
Both struc-tures contain the following sequential layers (top to bottom); otoconia, otoconial membrane and sensory epithelium (fig. 3). The maculae in mammals are covered with otoconia or statoconia, which are calcite crystals, a cal-cium carbonate structure with a specific weight of 2.95 grams per cubic centimeter (Parker, 1980). The shape of the otoconia is mostly hexagonal, but variations in shape exist. The size of the otoconia can vary from 3 to 30 µm and regional differences exist in both the utricle and the saccule (Lim et al. 1973).

Fig. 2. Macula utricle (A) and saccule (B); shape of the macula. Drawings; location of the striola (dotted line) and direction of the polarization of the haircells (arrows).
Besides calcium, the otoconia can also contain potassium, magnesium, copper and phosphorus (Lim, 1973; Chou et al, 1980; Campos et al, 1992; Crespo et al, 1993). A homogeneous ground substance is present in the crystal that often becomes condensed as a nucleus in the center of the otoconium (Anniko, 198-0). This substance, called the otoconia matrix, seems to be morphologically similar, if not identical to the substance of the under-lying layer, e.g. the otolith membrane (Lim, 1973).
At least in rodents, the formation of most otoconia occurs rapidly during a period of some days in the embryonic phase; for example, in rats otoconia are observed after the 16th embryonic day. (Salamat, 1980; Anniko, 1980; Anniko et al, 1987). It is hypothesized that the initial stages and duration of otoconia genesis are demar-cated by organic fibrils (muco-polysaccharides and mucoproteins) attached to the stereocilia. These fibrils are released by the secretory granules in the support-ing cells of the epithelial cell layer (An-niko, 1980; Kawamata and Igarashi, 1993). The fibrils later segment into presumptive units, away from the stereocilia. Segmentation away from the stereocilia allows otoconia flexibility from the beginning of formation. Such flexibility is further enhanced by the cementing substance between otoconia and the otolith membrane by strands of membrane material (Ross et al, 1987; Fermin, 1993). Once the nucleus, the basic unit, is formed the crystal grows by addition of calcite- to the surface, layer for layer (Ross and Peacor, 1975). The incorporation of calcium into the otoconia occurs as a directed flow from the surface of the macular epithelial cells (Anniko, 1980; Anniko et al, 1987). Inhibition of crystal growth occurs once a critical - calcium concentration in the endolymph and an adequate size of otoconial mem-bra-nous chambers are reached (Sánchez-Fernández and Rivera-Pomar, 1984).
The underlying structure is called the otolith or gelatinous membrane. Care must be taken by describing the otolith membrane because it is not clear how the structure of this membrane looks in vivo. The actual physical appearance, the filament size and precise arrangement of the two materials above the sensory epithelia are dependent upon preparation methods. This membrane seems to consist of two structurally distinct layers; a peripheral layer and a subcupular zone. The peripheral or upper layer supports the otoconia while the supbcupular zone, which is formed by long filamentous structures, extends down to the surface of the sensory epithelium (Lim, 1973; Ross et al, 1987; Takumida et al, 1992).
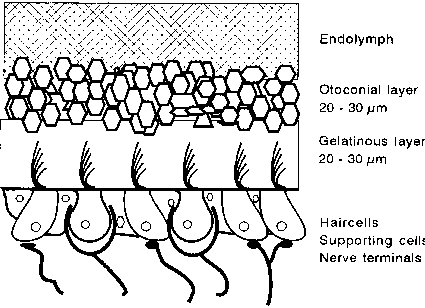
Fig. 3. Schematic cross-sectional view of the otolith organ.
Bundles of hairs, coming from the underlying haircells, are present in the otolith membrane. Each bundle consists of many small hairs, the stereocilia, and one longer hair, the kinocilium. The number of stereocilia varies from species to species but also varies in different sensory areas within one species. The longer stereocilia are located near the kinocilium. Kinocilia and some longer stereocilia are attached to the otolith membrane. The kinocilium is connected to specific stereocilia and the stereocilia are interconnected with each other through strands of macromolecules (tip links) running from the top of one stereocilium into the side of the tallest neighbour (Ross et al, 1987; Takumi-da et al, 1992). The kinocilium of mammals is composed of nine peripherally double fibrils of micro-tubules and one central double fibril. These fibrils are absent in the stereocilia that are packed with actin filaments, which are added to the stereo-cilia during growth (Shepherd 1994). The basal body of the kinocilium is located in a cuticle-free region, while the roots of the stereocilia are fixed in the cuticular plate of the haircells (Engström and Engström, 1981).
In the next layer, the sensory epithelium, the hair cells are present. Within the maculae utricle and saccule the hair cells are oriented in opposite directions on either side of the striola. The striola follow curved paths across the planes of the maculae so that there are hair cells responding effectively in all directions in the plane of the macula (Gresty et al, 1992). Two types of hair cells, with their bundles of hairs in the otolith membrane, can be identified. The type I cell is flask-shaped with a rounded lower end, whereas the type II cell has a more cylindric form (fig. 4).
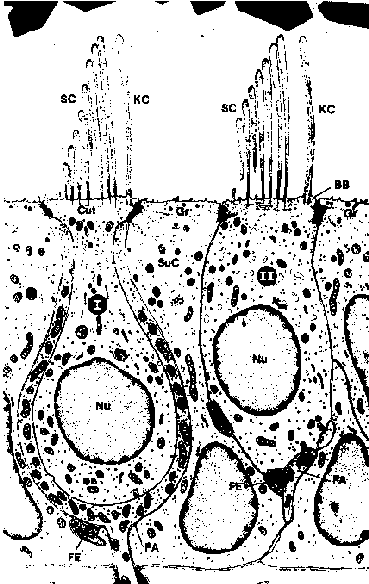
Fig. 4. Photo of type I hair cell (I) and type II hair cell (II) and supporting cells (SuC). BB: basal body; Cut: cuticular plate; FA: fasciculus afferens; FE: fasciculus efferens; Gr: granular material; KC: kinocilium; Nu: nucleus; SC: stereocilia (modified from Van het Schip, 1983).
Both types of hair cells synapse with calyces, or with calyceal collaterals that end as button-like swellings on type II hair cells. The type I haircell is surrounded by a thick membrane formed by the sensory cell and one calyx. Calyces serve to isolate the type I hair cell from the type II hair cells by enclosing them (Ross, 1988) which enables them to act as variable pass filters. These filters contribute a type of weighting in the circuitry. According to Engström and Eng-ström (1981) the main difference between both cells is that the type I hair cell is in direct contact only with one termination or calyx from an afferent nerve fiber at the lower portion of the hair cell, whereas the type II hair cell forms direct synaptic contact with both afferent and efferent cells in different locations along the cell membrane. The sensory layer also contains supporting cells, which are present in a higher number than sensory cells. Although differences in shape are common, most of these cells reach from the basement membrane to the epithelium surface were they have a small number of microvilli. The supporting cells are grouped around the type II hair cells and their nerve endings and around the calyx of the type I hair cells.
2.1.2 Physiology of the otolith organs
The otolith organs are responsible for the transduction of forces produced by linear accelerations, such as gravity. The neural output from these organs serve as an input signal to oculomotor reflexes, postural mechanisms and perceptual systems. Therefore, these organs play a role in generating compensatory eye movements in responses to linear acceleration of the head, and in changes in orientation and static postural functions with respect to gravity- (Gresty and Bronstein, 1992).
The otolith organs can be represented as mass-spring systems. Ross (1987), Tak-umida et al (1992) and Fermin (1993) suggest that the otoconia represent seismic mas-ses that are spring-loaded, through the otoconial membrane, to the kinocilia and some longer stereocilia. Even when the head is at rest the otoconia, because of their mass, exerts a force upon the underlying otoconial membrane and the cilia. This force is equal to the product of its mass and the gravitational force, which is on earth 9.80 m/sec2. During a change in linear acceleration the otoconia press on the cilia, thus bending them. The substance of the otolith membrane may function as a second (softer) spring, to dampen the system and help to isolate unwanted vibrations (Takumida et al, 1992). If displacement occurs only in a few cilia, all cilia will be displaced together as a bundle because of the connection through the tip-links between them. This connection supports the hypothesis for a dynamic interaction from the kinocilium to the stereocilia. The kinocilium is motile and leads the tuft in its responses to linear acceleratory forces. The location of the kinocilium relative to the stereociliary tuft deter-mines cell polarization: deflection of the stereocilia in the direction of the kinocilium excites the cell; deflection in the opposite direction inhibits it (Ross, 1988). The deflection of the bundle causes a receptor potential current flowing via the tip of the stereocilia to the cell body. The signal is further transmitted via the sensory fibers to the central vestibular nuclei where the integration with information from the other sensory systems takes place. Because the hair bundles are all oriented towards the striola, all possible stimuli orientations can be detected within the plane of the macula. Functionally, the striola defines a change in the polarization of cells of the sensory epithelium (Hunter-Duvar, 1983).
Ross (1985, 1988, 1993) suggests that the information concerning gravito-inertial forces is processed peripherally beginning at the hair cell level. A particular response is not related simply to the morphology of its own terminal field, but is also influenced by activity of its neighbours in the network, through shared type II cells. Regularity or irregularity of discharge pat-terns may be related, at least in part, to the fact that unmyelinated nerves and their calyces come into close physical contiguity in some areas, increasing the likelihood of electrical interaction between them. This would likely act to synchronize their discharges and make them more regular, where-as in other areas, individual nerves stay physically apart from one another. The hair cell responses are transmitted via the axons of the eight cranial nerve to the central vestibular nuclei of the brainstem.
2.2 The semicircular canals
2.2.1 Anatomy
The planes of the three semicircular canals, two vertical canals (canalis verticalis anterior and posterior) and one horizontal canal, are almost at right angles at each other. The information of the canals from each side of the head permit detection of all possible angular accelerations of the head. The canals are filled with endolymph. The relative length, diameter and shape of the three semicircular canals vary from species. It is suggested that in free-swimming and flying animals the canals are longer and of smaller diameter than in animals moving near the ground in a stable equilibrium (Lowenstein, 1974).
A widened part is found in each canal, the ampulla, which contains the crista ampullaris that is running across the ampulla about perpendicular to the plane of the canal. At the top of this septum, the sensory receptors are found. The receptor hair cells type I and II, identical to those from the otolith organs, contain the kinocilium and the stereocilia, which are oriented along the axis of each canal. The crista is covered by a cupula in which the cilia are en-sheathed. The cupula is a gelatinous fibrillar structure with a specific gravity resembling that of the labyrinthine endolymph, (Engström and Engström, 1981). The structure of the hair cells and the supporting cells are highly similar to those of the utricle and saccule (fig. 5).
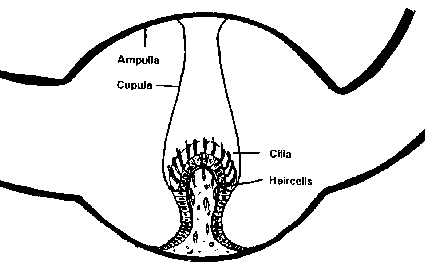
Fig. 5. Schematic view of the ampulla of a semicircular canal (modified from Parker, 1980).
2.2.2 Physiology
The three semicircular canals are designed to detect rotatory accelerations, resulting from circular motion that is measured by the change in rate of rotation. Rotation of the head affects one canal or a combination of the three canals, by causing movement of the cupula-endolymph system within the semicircular canal, which in turn causes a shearing force on the hairs projecting into the cupula. The bending of the hairs (stereocilia and kinocilium) relative to the hair cell body results in hyperpolarizing or depolarizing receptor potentials. The unmyelinated fibers of the primary vestibular afferents carry the potentials to the basement membrane of the neuroepithelium where the axon becomes myelinated. From here, the action potential travels along the axon to its cell body in the vestibular (Scarpa’s) ganglion and continues its way via myelinated post ganglionic fibers to cells within the central vestibular nuclei for further processing (Correia et al, 1981). During constant rotation the canals' fluid moves at the same rate as the body causing the cupula to retain its original position, whereas decreases or increases in acceleration causes new changes in the cupula's position thus new receptor potentials (Shepherd, 1994).
2.3 The central vestibular system
The central vestibular system has many connections to other parts of the brain and the rest of the body. Not only the vestibular system, but also other sensory systems, such as visual and the somatosensory systems, have access to the vestibular nuclei. Because of the complexity, only the major parts of this system and their projection areas are discussed.
The vestibular nerve signals arrive via the eight cranial nerve to the brain-stem. The brainst-em contains four vestibular nuclei: the superior (Bechterews' nucleus), the lateral (Deiters’ nucleus), the medial (nucleus of Schwalbe) and the inferior (Rollers' nucleus). Each nucleus consists of distinct cell groups with their own functions. Furthermore, cell groups can be distinguished lying within one of the major nuclei that have their own special cyto-architectonic features, such as the interstitial nucleus of the vestibular nerve, the group f (a collection of densely packed large cells in the inferior nucleus), and nucleus supravestibularis. The maculae utricles and saccules project mainly to the lateral and medial nucleus, while the semicircular canals project mainly to the medial and superior nucleus.
From the vestibular nuclei in the brain-stem fibers lead to three main projection systems; the vestibulo-spinal system, the vestibulo-ocular system and the vestibulo-cerebellar system. Collaboration exists between the vestibular nuclei of the two sides of the brainstem by means of efferent commissural connections (Precht, 1974).
The vestibulo-spinal system is necessary for adjusting body musculature to maintain orientation in space. A distinction can be made between head-neck muscles and limb-muscles. The reflexes of the head-neck act as a feedback system for stabilizing the head in space and link the movements of the head to those of the body. The lateral vestibular nucleus controls limb moto-neurons and the fibers are exclusively excitatory, the medial tract controls neck and trunk muscles and the fibers are both excitatory and inhibitory.
The vestibulo-ocular system controls the eye-movements in order to maintain a stable visual field image despite movements of the body or head. These eye-movements are called compensatory eye-movements and are controlled by the vestibulo-ocular reflexes. They occur when trying to focus at a point in the visual field when the head is rotating. The slow phase of the eye-movement is a slow movement of the eyes in the opposite direction of the rotation and is directed by the semicircular canal information. Torsional eye movements are contra movemen-ts of the eye which attempt to maintain their original position in space when the head is tilted. They are directed by the otolith organs (Par-ker, 1980).
The vestibulo-cerebellar system is involved in the control of muscle tone, balance and sensori-motor coordination by integrating the information coming from the visual, vestibular and somatosensory systems. The cerebellum not only receives vestibular information coming from the vestibular nuclei but also controls these nuclei by projections to these areas (Shepherd, 1994).