Chapter
B-II
Epidermal
growth factor induced expression of c-fos is influenced by altered gravity conditions.
R.P. de Groot, M.Sc.,
P.J. Rijken*, M.Sc., J. Boonstra*, Ph.D., A.J. Verkleij*, Ph.D., S.W. de Laat,
Ph.D and W.Kruijer, Ph.D.
ABSTRACT
Epidermal growth factor (EGF) activates
a well characterized signal transduction system in human A431 epidermoid carcinoma
cells, which leads to rapid and transient expression of the c-fos proto-oncogene.
In order to investigate the influence of altered gravity on EGF-induced signal
transduction, we have studied the EGF-induced c-fos expression under simulated
hypo- and hypergravity conditions. In this report we show that EGF-induced fos
expression is decreased under simulated hypogravity conditions, while hypergravity
has a stimulatory effect on EGF-induced fos expression. These results show that
the EGF-activated signal transduction system is influenced by gravity, and that
gravity exerts its effects already in the early phases of the signal transduction
cascade.
INTRODUCTION
In recent years the increased use of sounding
rockets and space shuttles for biological and biomedical experiments has strengthened
the idea that microgravity may effect cell growth and differentiation of both
prokaryotic and eukaryotic cellular systems (reviewed in ref.1). A striking
example of such effects has been given by Cogoli and coworkers. Based on the
initial observation that space shuttle crew members showed a temporary immunodeficiency,
they have demonstrated that the activation of cultured lymphocytes by the plant
lectin Concanavalin A (Con A) is almost totally depressed in microgravity (2),
whereas it is enhanced at 10xg (3). The molecular mechanisms underlying these
gravity effects are unclear as yet. Con A exerts its mitogenic activity in lymphocytes
by eliciting a cascade of signal transduction events, normally evoked by the
interaction of endogenous mitogens with their specific surface receptors, such
as polypeptide growth factors. Among these factors the action of epidermal growth
factor (EGF) is probably characterized in most detail. A particular suitable
and extensively studied target cell for EGF is the A431 human epidermoid carcinoma
cell, that expresses an extraordinary large number of EGF receptors ( 2.106/cell)
(4). Binding of EGF to these receptors results in the immediate activation of
a well characterized signal transduction system that transduces this signal
to the nucleus, causing altered patterns of gene expression that may eventually
lead to DNA synthesis and cell division, depending on the cell type involved
(5). The induction of the so-called immediate early genes, like the proto-oncogene
c-fos, is the earliest detectable nuclear indication of a normally functioning
signal transduction cascade (6). The EGF-induction of this gene thus constitutes
an ideal assay to monitor possible gravity effects on receptor-mediated signal
transduction in general. Here we show that hypogravity, simulated in a fast
rotating clinostat, decreases EGF-induced fos expression, while hypergravity
increases EGF-induced fos expression. This study is the first to show an effect
of altered gravity conditions on gene expression in mammalian cells.
MATERIALS AND METHODS
Cells
Human A431 epidermoid carcinoma cells were
cultured in Dulbecco's Modified Eagle's Medium supplemented with 7.5% foetal
calf serum (FCS, Flow Labs, Irvine, Scotland). One hour prior to stimulation,
the medium was changed for DMEM- Hepes without serum. For clinostat experiments,
cells were cultured on thermanox coverclips (Lux Scientific Corporation, Newbury
Park, CA). For centrifuge experiments, cells were cultured in 5 mm culture dishes.
EGF (Collaborative Research, Waltman, MA) was added at 50 ng/ml at 370C,
unless indicated otherwise.
RNA isolation and Northern blotting
Total cellular RNA was isolated by the guanidine
isothiocyanate/caesium chloride method of Chirgwin et al (7). RNA was denatured
for 10 min at 680C in 50% (v/v) formamide, 2.2 M formaldehyde, 20
mM MOPS pH 7.0, 5 mM sodium acetate, 1 mM EDTA, separated through 0.8% agarose/2.2
M formaldehyde gels, and subsequently transferred to nitrocellulose filters
(BA 85, Schleicher & Schuell) in 20X SSC (1xSSC contains 0.15 M NaCl and
0.015 M sodium citrate, pH 7.0). RNA was immobilized by baking at 800C
for 2 hr under vacuum. Hybridizations were performed in 50% formamide, 5X SSC,
50 mM sodium phosphate pH 6.8, 10 mM EDTA, 0.1% NaDodSO4, 0.1 mg of sonicated
salmon sperm DNA per ml, 2X Denhardt solution (1X Denhardt solution contains
0.02% bovine serum albumin, 0.02% ficoll, 0.02% polyvinylpyrrolidone) at 420C
overnight. The fos probe consisted of a 1.0 kb PstI fragment derived from pfos-1
(9), 32P-labeled using a multiprime DNA labeling kit (Amersham).
After hybridization and washing (final wash in 0.1xSSC at 6000C),
the filters were exposed to Kodak XAR-5 film at -700C using intensifying
screens.
RNAse protection analysis
RNAse protection analysis was performed
according to Melton et al. (8). 2-5 µg of total cellular RNA was hybridized
to a 32P-labeled complementary RNA probe, derived from a 121 bp AvaI-HincII
fragment located between positions 297 and 418 of the human c-fos gene (12).
After RNase digestion of the resulting RNA-RNA hybrid, a protected fragment
of 110 nucleotides is indicative for expression of c-fos mRNA.
Clinostat and centrifuge
For clinostat experiments, a portable fast
rotating clinostat developed in cooperation with CCM (Centre for Construction
and Mechanisation, Nuenen, The Netherlands) was used. All experiments were performed
at 60 rotations per minute (rpm). For centrifuge experiments, a slightly modified
type Hettich centrifuge equipped with a swing-out rotor for microtiter plates
was used. All experiments were performed at 600 rpm (10xg), unless indicated
otherwise.
RESULTS
Induced expression of the c-fos gene.
Treatment of subconfluent cultures of A431
cells with EGF leads to a rapid, transient increase in c-fos mRNA levels (fig
1A). A 20-fold increase of fos mRNA level is observed after 10 min, while a
maximal increase (50-fold) occurs after 30 min. Fos induction by EGF is transient
and has returned to pre-stimulation levels after 2-2.5 hr. When investigated
by the more sensitive RNA protection analysis, an increase of fos mRNA can already
be detected 3-6 min after EGF treatment (fig 1B). In the presence of the protein
synthesis inhibitor cycloheximide, EGF leads to a higher and longer lasting
increase in fos expression (data not shown), by blocking the synthesis of proteins
responsible for repression of fos transcription and normal degradation of fos
mRNA.
To show that the induction of fos mRNA is
a specific process, the doses dependency was investigated. Subconfluent A431
cells were stimulated for 30 min with different concentrations of EGF ranging
from 0.01 up to 100 ng/ml. RNA was isolated and analyzed by Northern blotting.
As shown in fig. 2A, the induction of the fos gene is dependent on EGF concentration.
Half maximal stimulation occurs at 1 ng/ml, while maximal induction of the fos
gene is achieved by 10-20 ng/ml EGF. The EGF-induced fos expression is also
temperature dependent. At 40C no induction of this gene by EGF can
be detected, while maximal fos induction was found at 30-370C (fig
2B).
Induction of the fos gene in A431 cells
is not specific for EGF. As shown in fig 3, various agents such as phorbol esters
(TPA), Ca2+ ionophores (A23187 and ionomycin), and mitogenic neuropeptides
(bradykinin, histamin and bombesin) are able to induce fos expression, although
with different efficiencies, when applied at excess concentrations. TPA and
A23187 mimic aspects of the signalling cascade initiated by EGF or mitogenic
neuropeptides (5). Ionomycin is the most potent inducer of fos expression in
A431 cells. These results show that induction of the proto-oncogene c-fos is
an important and common element in the signal transduction mechanisms evoked
by growth factors and mitogenic neuropeptides.
Effects of gravity changes on EGF-induced
fos expression
The possible effects of altered gravity
conditions on signal transduction were studied by determining the EGF-induced
rise in c-fos mRNA levels under simulated hypogravity and hypergravity conditions.
For this purpose, A431 cells were cultured on Thermanox coverslips for 48 hr
and then mounted on a fast rotating clinostat. After rotation for 2 hr (60 rpm)
at 370C, EGF was added at 50 ng/ml for 15 min. Total RNA was isolated,
and 2 µg of RNA was hybridized to a 32P-labeled fos antisense RNA
probe. Fos mRNA expression was measured by RNase protection (see materials and
methods). Fig 4 shows that while constitutive fos mRNA levels are not changed
by simulated hypogravity conditions (compare lanes 1g- to 0g-), EGF-induced
fos expression was slightly depressed under hypogravity conditions (compare
lanes 1g+A to 0g+). This experiment was repeated five times with identical results.
By scanning the autoradiographs and plotting the 0g/1g ratio, a 20% depression
of EGF-induced fos expression was evident under hypogravity conditions (fig
5). Shortening the rotation time to 15 min still showed the same effect, although
less pronounced (data not shown).
When the EGF-induced fos expression was
investigated under hypergravity conditions, the opposite effect was found. Cells
were cultured in 5 mm tissue culture dishes for 48 hr, and then placed in a
centrifuge operated at 600 rpm (10g) for 2 hr. After addition of EGF (50 ng/ml
at 370C), centrifugation was continued for 15 min before cells were
lysed and the RNA was isolated. Fig 4 shows, that hypergravity slightly increased
the EGF-induced fos expression (compare lanes 10g+ to 1g+B), while no effect
was observed on the constitutive expression of the fos gene (compare lanes 10g-
to 1g-). Repeating this experiment three times confirmed these results. Plotting
the 10g/1g ratio of these experiments shows that hypergravity increases the
EGF-induced fos expression by 18% (fig 5). When higher gravity values were applied,
no further increase in fos mRNA levels was found (data not shown). These results
clearly show that EGF-induced fos expression in A431 cells is sensitive to gravity
changes, and are encouraging for further exploiting this system to study gravity
dependent modulations of signal transduction in mammalian cells.
DISCUSSION
The induction of the c-fos proto-oncogene
is a rapid nuclear response following activation of the signal transduction
cascade by extracellular factors, and is therefore a good indicator to study
the influence of gravity changes on this proces. Our results show that simulated
microgravity decreases EGF-induced fos expression, while hypergravity leads
to an increase in fos mRNA levels. This suggests that gravity exerts its effects
already in the early phases of the signal transduction cascade. This notion
is strengthened by our recent observation that simulated gravity changes have
an effect on EGF-induced alterations in cell morphology, which is also detectable
within minutes of growth factor addition (13).
Our results are in agreement with investigations
by Cogoli et al., showing that gravity alters mitogen-induced signal transduction
mechanisms (2,3). It would therefore be interesting to see if Con A-induced
fos expression in lymphocytes is also influenced by altered gravity conditions.
Although Cogoli et al. have shown, that the reaction of the cell to a change
of the g-environment does not follow general rules, but rather depends on the
cell type (10), influencing the level of expression of the fos gene might be
a common way for gravity to exert its effect on cell proliferation and differentiation.
In this respect, it is noteworthy to mention that cells which are induced to
change their growth state by various agents, and thus are likely to express
the fos gene, seem to be more sensitive to gravity changes than normal proliferating
or resting cells (A. Cogoli, personal communication).
Obviously, the molecular target(s) for the
gravity effects on signal transduction are as yet unknown. As a possible candidate
one could think of the cytoskeleton. This structure determines cell morphology
and is subject to alteration upon EGF addition (reviewed in ref. 11). Recent
experiments by van Bergen en Henegouwen, however, have linked the cytoskeleton
actin filaments to the regulation of c-fos induction. Blocking the polymerization
of actin filaments with cytochalasin B significantly decreased EGF-induced fos
levels. This effect is not completely surprising, since the EGF-receptor is
known to be associated with the actin filaments. It might well be that altered
gravity conditions somehow modulates the cell's cytoskeleton, thereby causing
a change in EGF-induced fos expression. Further study of the EGF-induced signal
transduction in A431 cells under real microgravity conditions will hopefully
give more insight in the way gravity influences mammalian cell proliferation
and differentiation.
Acknowledgements
The authors would like to thank A. Cogoli
and W. Briegleb for inspiring discussions, and J. den Hertog for critical reading
of this paper. This work was supported by the Space Research Organisation Netherlands
(SRON).
REFERENCES
1 - Gmunder FK, Cogoli A. Cultivation of
single cells in space. App. Microgravity Tech. 1988;1:3:115-22.
2 - Cogoli A, Tschopp A, and Fuchs-Bislin
P. Cell sensitivity to gravity. Science. 1984;225:228-30.
3 - Lorenzi G, Fuchs-Bislin P, and Cogoli
A. Effects of hypergravity on "whole-blood" cultures of human lymphocytes. Aviat.
Space Environ. Med. 1986;57:1131-35.
4 - Fabricant RN, De Larco JE, and Todaro
GJ. Nerve growth factor receptors on human melanoma cells in culture. Proc.
Natl. Acad. Sci. USA. 1977;74:565-69.
5 - Carpenter G. Receptors for epidermal
growth factor and other polypeptide mitogens. Ann. Rev. Biochem. 1987;56:881-914.
6 - Kruijer W, Cooper JA, Hunter T, and
Verma IM. Platelet-derived growth factor induces rapid but transient expression
of the c-fos gene and protein. Nature.1984;312:711-16.
7 - Chirgwin JM, Przybyla AE, MacDonald
RJ, and Rutter WJ. Isolation of biologically active ribonucleic acid from sources
enriched in ribonuclease. Biochemistry. 1979;18:5294-99.
8 - Melton DA, Krieg PA, Rebagliatie MR,
Maniatis T, Zinn K, and Green MR. Efficient in vitro synthesis of biologically
active RNA and RNA hybridization probes from plasmids containing a bacteriophage
SP-6 promotor. Nucleic Acids Res.1984;12:7025-56.
9 - Curran T, Peters G, van Beveren C, Teich
NM, and Verma IM. FBJ murine osteosarcoma virus: Identification and molecular
cloning of biologically active proviral DNA. J. of Vir. 19821:674-82.
10- Lorenzi G, Bechler B, Cogoli M, Cogoli
A. Gravitational effects on mammalian cells. The physiologist. 1988;31:1:S144-47.
11- Boonstra J, Defize LHK, van Bergen en
Henegouwen PMP, de Laat SW, and Verkleij AJ. Characteristics of the epidermal
growth factor receptor. In: Evangelopoulous AE, Snoek GT, Wirtz KWA, and Changeux
JP, eds. Receptors, membrane transport and signal transduction. Heidelberg:
Springer Verlag, 1989; in press.
12 - van Straaten F, Muller R, Curran T,
van Beveren C, and Verma IM. Complete nucleotide sequence of a human c-onc:
Deduced amino acid sequence of the human c-fos protein. Proc. Natl. Acad.
Sci. USA. 1983;80:3183-87.
13- Rijken PJ, De Groot RP, Briegleb W,
Kruijer W, Verkleij AJ, Boonstra J, and de Laat SW. Epidermal growth factor
induced cell rounding is sensitive to simulated weightlessness. Aviat. Space
Environ. Med., 1990, in press.
FIGURE LEGENDS
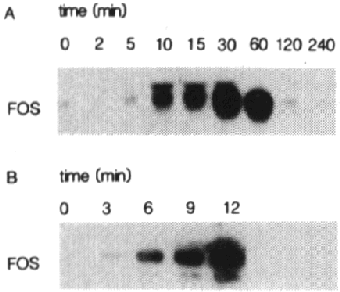
Fig. 1 - Induction of c-fos mRNA by EGF in A431 cells.
A - Subconfluent A431 cells were stimulated with
EGF for the indicated times. 15 µg of total RNA was loaded in each lane and
subjected to Northern blotting analysis. Filters were hybridized to a radiolabeled
fos probe and processed as described in materials and methods. Autoradiography
was for 16 hours.
B - Subconfluent A431 cells were treated with EGF
for the indicated times. 5 µg of total RNA was hybridized to a 32P-labeled
fos antisense RNA probe followed by digestion of the resulting RNA-RNA hybrids
with RNase A. The protected RNA hybrids were loaded onto a 6% polyacrylamide
gel, run at 2.5 V/cm, dried and visualized as described in materials and methods.
Autoradiography was for 14 hours.
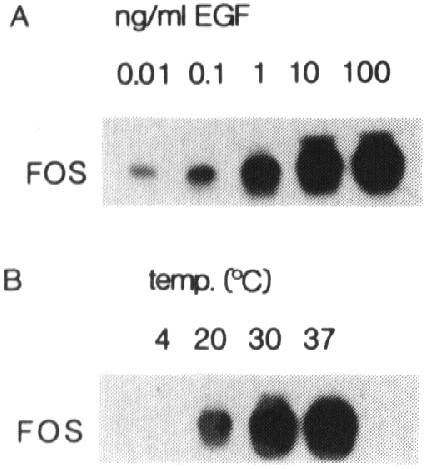
Fig 2 - Fos induction by EGF is concentration and temperature dependent.
A431 cells were treated for 30 min with various
concentrations of EGF (A) or treated with 50 ng/ml EGF for 30 min at different
temperatures (B). 15 µg of total RNA was loaded in each lane and analyzed as
described in the legend of fig. 1A.
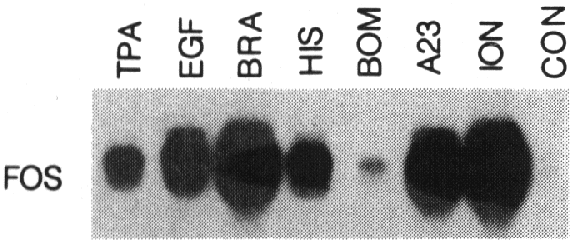
Fig 3 - Various agents induce fos mRNA expression.
A431 cells were treated for 30 min with phorbol
12-myristate 13-acetate (TPA, 100 ng/ml), EGF (50 ng/ml), bradykinin (BRA, 2.5
µmM), histamin (HIS, 0.5 mM), bombesin (BOM, 1 µM),
A23187 (A23, 2 µM)
or ionomycin (ION, 5 µg/ml).
15 µg of RNA was loaded
in each lane and subjected to Northern blotting analysis as described in the
legend of fig 1A.
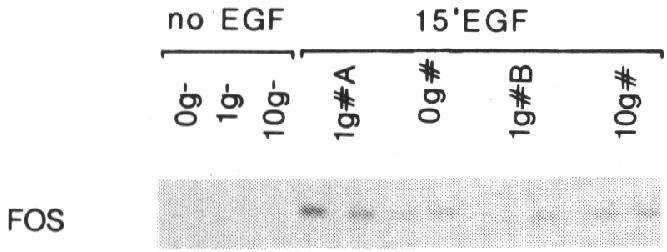
Fig 4 - Effect of gravity changes on EGF-induced fos expression.
Subconfluent A431 cells were cultured for 2 hr
in a fast rotating clinostat (0g), a fixed clinostat tube (1gA), a centrifuge
(10g) or a normal culture dish (1gB). EGF (*) was added for 15 min. Control
samples did not receive EGF (-). 2 µg
of RNA was analyzed by RNase protection as described in the legend of fig 1B.
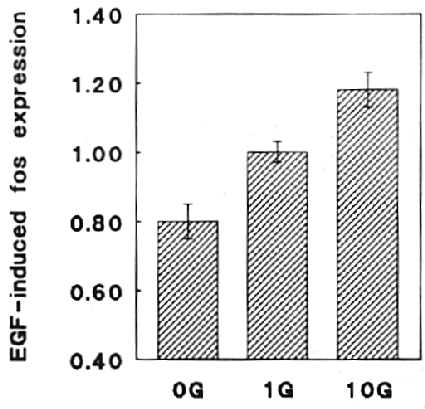
Fig 5 - Gravity changes influence EGF-induced fos expression
Fos expression in 4 different centrifuge experiments
(10g) and 5 different clinostat experiments (0g) was analyzed and compared to
the 1g control experiments. The diagram shows the ratio of 10g over 1g and 0g
over 1g experiments, respectively. The dotted lines represent the ratios of
control experiments.




