Chapter B-III
Microgravity decreases
c-fos induction and SRE activity
Rolf P. de Groot, Philip J. Rijken, Jeroen den Hertog, Johannes
Boonstra, Arie J. Verkleij, Siegfried W. de Laat and Wiebe Kruijer.
SUMMARY
Several studies have shown that altered gravity conditions
influence mammalian cell growth and differentiation. The molecular mechanisms
underlying these effects however remain relatively obscure. In this paper we
show that microgravity reached in a sounding rocket strongly decreases epidermal
growth factor (EGF) induced expression of the proto-oncogenes c-fos and
c-jun, that are both implicated in the regulation of proliferation and
differentiation. Decreased activity of the serum response element (SRE), present
in the c-fos promoter - enhancer region, is probably responsible for
the decrease in EGF-induced c-fos expression. In addition, we show that
gravity alterations differentially modulate distinctive signal transduction
pathways, indicating that gravity dependent modulations of mammalian cell proliferation
are unlikely to be caused by a-specific stress responses of the cell.
INTRODUCTION
During the last decade life science research under altered
gravity conditions has become a field of increasing interest. Although the mechanism
of gravity sensation in plant cells is relatively well understood (Halstead
and Dutcher, 1987), the knowledge of effects of altered gravity on animal cells
is still rather limited. Extensive studies of gravity effects on mammalian cells
were performed by Cogoli and coworkers, who showed that mitogenic stimulation
of human lymphocyte cultures by the plant lectin concanavalin A (con A) is almost
completely depressed under microgravity (Cogoli et al., 1984, Bechler et al.,
1986), and that hypergravity enhances the stimulation of lymphocytes by con
A (Lorenzi et al., 1986) as well as the proliferation of other mammalian cells
(Tschopp and Cogoli, 1983).
How gravity alterations affect cell proliferation remains
to be established. Possible clues for the underlying molecular mechanisms have
been obtained from recent studies indicating that gravity may exert its effect
by modulating the expression of growth regulatory genes, such as proto-oncogenes.
Hypergravity appears to stimulate the proliferation of human HeLa cells through
a reduction of the G1 phase duration, and enhances the expression of the c-myc
proto-oncogene in these cells (Kumei et al., 1989), a gene whose product is
known to play an important role in cellular proliferation (Kelly and Siebenlist,
1986). We have demonstrated that in human A431 cells EGF induced expression
of the c-fos proto-oncogene is significantly decreased under simulated
weightlessness conditions, whereas it is enhanced by hypergravity (de Groot
et al., 1990a). In addition we found that EGF induced cell rounding is modulated
by gravity changes (Rijken et al., 1990). Taken together these observations
suggest that gravity may affect the intracellular signalling pathways activated
by mitogenic stimuli such as growth factors, ultimately resulting in the modulation
of proto-oncogene expression.
The gene products of the c-fos and c-jun proto-oncogene
family are known for their prominent role in cell proliferation (reviewed in
Imler and Wasylyk, 1989) and differentiation (Müller and Wagner, 1984;
de Groot et al., 1990b). Their expression is usually rapidly induced by growth
factors and can be induced also by a variety of agents that bypass the receptor
and mimmick the partial activation of signal transduction pathways (for a review,
see Verma and Sassone-Corsi, 1987). Examples of these latter are phorbol esters
(e.g. TPA), Ca2+ (e.g. A23187) and agents that raise the intracellular
concentration of cAMP (e.g. forskolin).
In this study we demonstrate that in A431 cells EGF induced
c-fos and c-jun expression is strongly repressed in real microgravity,
probably as a result of changes in promoter-enhancer activity. In addition we
show that simulated microgravity differentially modulates distinctive signal
transduction pathways, since under these conditions EGF and TPA induced c-fos
expression is decreased whereas A23187 and forskolin induced c-fos expression
is not.
MATERIALS AND METHODS
Cells and plasmids
Human A431 epidermoid carcinoma cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 7.5% foetal calf serum (FCS).
One to four hours prior to stimulation, the medium was replaced for DMEM-Hepes
without serum. As probe for Northern hybridization studies a 0.8 kb Pst1 fragment
of v-fos (Curran et al., 1982) was used. Chloroamphenicol acetyltransferase
(CAT) reporter plasmids including pSV2CAT have already been described (Gorman
et al., 1982). The thymidine kinase promoter from herpes virus (positions -109/+57)
was fused to the CAT structural gene and used as a background for the analysis
of SRE sequences in the transfection experiments. SRE/DSE3tkCAT is
described elsewhere (de Groot et al., 1990c).
RNA isolation and Northern blotting
Total cellular RNA was isolated by the guanidine isothiocyanate-caesium
chloride method (Chirgwin et al., 1979). 15 µg of total RNA was denatured for
10 min at 680C in 50% (v/v) formamide, 2.2 M formaldehyde, 20 mM
3-(N-morpholino)propanesulfonic acid (MOPS) pH 7.0, 5 mM sodium acetate, 1 mM
ethylenediaminetetraacetic acid (EDTA), separated through 0.8 % agarose /2.2
M formaldehyde gels, and subsequently transferred to nitrocellulose filters
(BA 85, Schleicher & Schuell) in 20X standard sodium citrate (SSC). RNA
was immobilized by baking at 800C for 2 hr under vacuum. Hybridization
was performed in 50% formamide, 5X SSC, 50 mM sodium phosphate pH 6.8, 10 mM
EDTA, 0.1% NaDodSO4, 0.1 mg of sonicated salmon sperm DNA per ml, 2X Denhardt
solution (1X Denhardt solution contains 0.02% bovine serum albumin, 0.02% ficoll,
0.02% polyvinylpyrrolidone) at 42oC overnight. 32P-labeled
probes were generated using a multiprime DNA labeling kit (Amersham). After
hybridization and washing, filters were exposed to Kodak XAR-5 film at -70oC
using intensifying screens.
RNase Protection Analysis
RNase protection analysis was performed according to Melton
et al. (1984). 1-2 µg of total cellular RNA was hybridized to 32P-labeled
complementary RNA probes derived from the human c-fos gene (Van Straaten
et al., 1983), the human c-jun gene (Angel et al., 1988) and the human
.²-2-microglobulin gene (Suggs et al., 1981). After RNase digestion of the single
strand transcripts, protected fragments of 110, 155 and 80 nucleotides are indicative
for expression of c-fos, c-jun and .²-2-microglobulin respectively.
DNA transfection and transient expression assays
A431 cells were plated in DMEM-Bic/7.5% FCS at 4.106
cells per 75 cm2 tissue culture flask 24 hrs prior to transfection.
Two hours before transfection, the flasks recieved fresh medium. Cells were
incubated for 20-24 hrs with calcium phosphate precipitated DNA's (10-20 µg
plasmid per flask), followed by trypsinization of the cells and replating on
clinostat coverslips. 24 hours later, the medium was replaced for DMEM-Bic without
FCS. After incubation for 2 hours, the cells were mounted in clinostat tubes,
rotated and stimulated with or without EGF. After harvesting the cells and measuring
protein concentrations, CAT activity was determined. 20 µg of cell extract was
used for CAT assays (2 µg for pSV2CAT transfected cells). 1 µg of pSV2Apap DNA
was always included to serve as internal control to correct for possible variations
in the transfection efficiency. PAP assays were performed as described by (Henthorn
et al., 1988). CAT activity was determined as described (Gorman et al., 1982)
and was quantitated by liquid scintillation counting of TLC plate 14C
spots.
Clinostat experiments
For clinostat experiments, a portable fast rotating clinostat
developed in cooperation with CCM (Centre for Construction and Mechanization,
Nuenen, The Netherlands) was used. All experiments were performed at 60 rotations
per minute at 37oC.
MASER-3 sounding rocket experiment
A431 cells were cultured on coverslips and mounted into
the CIS-1 plunger box experiment units (CCM, Nuenen, see Fig. 1). The experiment
units were assembled in boxes and loaded in the CIS-1 module (Dutch Space (former Fokker Space) and
Systems, Amsterdam, The Netherlands) in the payload of the rocket. The temperature
of the experiment units remained 37oC during the whole experiment
due to active temperature control of the experiment boxes. After microgravity
was reached in the rocket, the cells were stimulated with EGF (100 ng/ml) or
medium alone (-EGF) by activation of plunger A (see fig. 1). After 6 minutes
the cells were washed with PBS (plungers B and D) and lyzed in guanidine isothiocyanate
(plunger E). After recovery of the payload, RNA was isolated as described above.
RESULTS
Previously we have shown that EGF-induced expression of
the c-fos proto-oncogene is significantly decreased under simulated hypogravity
conditions (de Groot et al., 1990a). To investigate whether this effect also
occurs in real microgravity, we performed an experiment in the CIS-1 module
flown on the MASER-3 sounding rocket (Swedish Space Cooperation, April 1989).
A431 cells were cultured in CIS-1 plunger box experiment units (Fig. 1) and
stimulated with EGF (100 ng/ml) or medium alone (-EGF) for 6 min. after microgravity
was reached in the rocket. Following recovery of the experiment units, RNA was
isolated from the lyzed cells and analyzed for c-fos transcripts by RNase
protection. As shown in figure 2, no c-fos transcripts were detected
in unstimulated (-EGF) cells neither in the 1-G control experiment (ground),
that was performed simultaneously with the flight experiment, nor in the microgravity
samples (flight). By contrast, the 1-g samples that were stimulated with EGF
for 6 minutes showed strong expression of the c-fos gene, which was decreased
by about 50% in the cells that were treated with EGF in microgravity.
Since the product of the c-fos gene is known to form
a functional heterodimeric complex with the product of the c-jun proto-oncogene
(reviewed in Busch and Sassone-Corsi, 1990), the expression of the c-jun
gene was studied. As shown in figure 2, c-jun expression was not detectable
in unstimulated cells, whereas it was decreased by about 50% in the EGF treated
flight sample as compared to EGF treated 1-g control cells. Expression of the
.²-2-Microglobulin gene, a gene that is not modulated by EGF treatment, was
constant in all flight and ground samples, indicating that the observed decrease
in expression of c-fos and c-jun is specific for EGF induced signal
transduction in these cells.
The induction of c-fos by EGF is mediated by the
serum response element (SRE) present in the 5' regulatory region of the c-fos
gene (Gilman et al., 1986; Treisman, 1986; Greenberg et al., 1987). This sequence
binds at least three different regulatory proteins, of which p67-SRF (serum
response factor) is probably mediating the effects of serum and EGF on c-fos
expression (Treisman, 1987). To assess whether the observed effects of microgravity
on EGF induced c-fos expression are caused by a modulation of SRE activity,
we coupled three copies of the c-fos SRE to a heterologous promoter (HSV
thymidine kinase, TK) and the bacterial chloroamphenicol acetyl transferase
(CAT) gene. A431 cells were transfected with this construct (named FOS in figure
3) and replated on clinostat slides after 24 hours. The slides were mounted
on a fast rotating clinostat after attachment of the cells for 24 hours and
rotated at 60 rpm for another 2 hours. Hereafter, the cells were either treated
with EGF (100 ng/ml) or medium alone for 8 hours. Collection of the cells and
assaying for CAT activity was performed as described in materials and methods.
As shown in figure 3A, no CAT activity was detected in cells treated with medium
alone, while treatment with EGF leads to a strong enhancement of CAT activity.
Comparison of EGF treated cells that were rotated on the clinostat (OG) and
cells cultured in fixed clinostat tubes (1G) shows that simulated hypogravity
decreased EGF-induced SRE activity. The activity of the SV40 promoter, that
is transcriptionally active in these cells, was not modulated by simulated hypogravity,
indicating that the decrease is specific for EGF induced SRE activity. Quantification
of CAT activity of 4 independent experiments shows that EGF induced SRE activity
(FOS in figure 3B), but not the activity of the SV40 promoter, is decreased
by about 30% under simulated hypogravity conditions (Fig. 3B). These results
show that the observed decrease in EGF induced c-fos expression is most likely
caused by a decrease in SRE activity.
A variety of different signal transduction pathways lead
to rapid increase of c-fos expression (reviewed in Verma and Sassone-Corsi,
1987). To examine if the observed modulations of c-fos expression by
gravity changes are specific for EGF induced signal transduction, we studied
the effects of simulated hypogravity on c-fos expression induced by the
phorbol ester TPA, the Ca2+ ionophore A23187 and the activator of protein kinase
A, forskolin. Cells were cultured for 40 hours on clinostat slides before mounting
on a fast rotating clinostat. After rotation at 60 rpm for 2 hours, the cells
were treated with EGF (100 ng/ml), TPA (100 ng/ml), forskolin (10 µM), A23187
(2.5 µM) or medium alone (CON) for 15 minutes. RNA was isolated and analyzed
for c-fos expression by Northern blotting. As shown in figure 4A, both
EGF- and TPA-induced c-fos expression was decreased under hypogravity
conditions (OG in the figure), whereas forskolin- and A23187-induced c-fos
expression remained constant. Scanning of the autoradiographs of 4 independent
experiments shows that both EGF and TPA induced c-fos expression are
significantly decreased in hypogravity (25% and 30% resp.), while no significant
differences were found with A23187 or forskolin. These results clearly demonstrate
that only a subset of signal transduction pathways that lead to c-fos
induction is sensitive to gravity alterations, and are encouraging for further
exploration of the effects of altered gravity conditions on mammalian cell proliferation
and intracellular signal transduction.
DISCUSSION
Life science research under altered gravity conditions is
important in determining the capacity of cells and organisms to cope with the
problems of gravitational stress. Moreover it might lead to clues on the functioning
of cells and organisms under normal gravity conditions. In this report we show
that EGF induced expression of the c-fos and c-jun proto-oncogenes
is severely decreased in real microgravity, extending our results from previous
studies in simulated hypogravity (de Groot et al., 1990a). At least for the
c-fos gene this effect is probably caused by decreased activity of the
SRE located in its 5' regulatory sequences. Studying other agents that induce
c-fos expression clearly shows that only a subset of signal transduction
pathways is sensitive to gravity changes.
A recent study has shown that hypergravity caused by centrifugation
enhances the expression of the c-myc proto-oncogene in proliferating
HeLa cells in the abscence of EGF (Kumei et al., 1989). Although no changes
in c-fos expression were found in these cells, our results are probably
in agreement with this study since we only detect changes in c-fos expression
in cells stimulated with growth factors or phorbol esters and not in unstimulated
resting (no FCS, see fig. 2 and 4A) or proliferating cells (7.5% FCS, unpublished
data). In addition we previously showed an enhancement of c-fos expression
in hypergravity in EGF treated, but not in untreated A431 cells (de Groot et
al., 1990a). The molecular basis of the differences between c-fos and
c-myc sensitivity to gravity changes remains to be determined, but may
involve the high constitutive expression of c-myc as opposed to the very
low basal level expression of c-fos in unstimulated cells.
EGF induced c-fos expression is mediated by the SRE
present in the c-fos promoter-enhancer region (see fig. 5 and Gilman
et al., 1986; Treisman et al., 1986; Greenberg et al., 1987). This sequence
binds at least three different proteins, of which p67-SRF is thought to mediate
the effects of serum and EGF on c-fos expression (Treisman et al., 1987).
Treatment of cells with EGF or serum leads to rapid phosphorylation of SRF,
a process likely to be important in the transcriptional activation of c-fos
(Prywes et al., 1988). Our results demonstrate that activation of the SRE by
EGF is decreased under simulated hypogravity conditions, and suggest that this
decrease is the basis for the observed effects of microgravity of EGF induced
c-fos expression. In this respect it is worthwile to mention that TPA
induced c-fos expression, a process that we show to be decreased in hypogravity,
is also mediated by the SRE (see fig. 5 and Gilman et al., 1988; Stumpo et al.,
1988). By contrast, forskolin and A23187 effects on c-fos expression
are not sensitive to gravity changes, while these agents exert their effects
on c-fos expression through regulatory sequences distinct from the SRE
(see fig. 5 and Fisch et al., 1987; Gilman et al., 1988). The mechanism by which
SRE activity is decreased under hypogravity conditions remains to be determined,
but one could speculate that EGF induced phosphorylation of p67-SRF might somehow
be modulated by gravity changes.
A variety of different agents induce expression of the c-fos
proto-oncogene. Our results show that EGF and TPA, but not forskolin and A23187
induced c-fos expression is decreased in hypogravity. EGF exerts its
effects through binding to its plasma-membrane located receptor followed by
the activation of an intracellular signal transduction cascade (reviewed in
Carpenter, 1987). Interestingly, activation of protein kinase C (PKC), the receptor
for the phorbol ester TPA, is a part of this signal transduction cascade (fig.
5). This suggests that PKC activity, or the activity of a downstream target
of PKC might be sensitive to gravity alterations. By contrast, both EGF and
A23187 treatment leads to a rise in intracellular Ca2+ (fig. 5), suggesting
that this process or its downstream targets are probably not influenced by gravity
changes. Forskolin raises the intracellular cAMP concentration via activation
of protein kinase A (PKA). Until now, no second messengers are identified that
are shared by both EGF and forskolin induced signal transduction (fig. 5), further
implicating PKC or one of its targets in gravity dependent modulations of mammalian
signal transduction.
The products of the c-fos and c-jun genes
are known to form functional heterodimeric complexes (reviewed in Busch and
Sassone-Corsi, 1990), and are implicated in the control of proliferation (reviewed
in Imler and Wasylyk, 1989) and differentiation (Müller and Wagner, 1984;
de Groot et al., 1990b). Our study shows that EGF induced expression of these
genes is strongly reduced in microgravity. Since a number of studies have shown
gravity dependent modulations of mammalian cell proliferation and differentiation
(Cogoli et al., 1984; Bechler et al., 1986; Lorenzi et al., 1986; Duke, 1983),
our findings suggest that gravity dependent effects on c-fos and c-jun
expression are likely to be of major importance for the cells reaction to gravitational
stress. Further study of the regulation of these and other regulatory genes
will hopefully lead to a more complete understanding of the way gravity exerts
its effects on mammalian cells.
ACKNOWLEDGEMENTS
The authors would like to thank all the people involved
in the CIS-1 experiment on the MASER-3 sounding rocket. This work was supported
by grants from the Space Research Organization Netherlands (SRON).
REFERENCES
Angel, P., Allegretto, E.A., Okino, S.T., Hattori, K., Boyle,
W.J., Hunter, T. & Karin, M. (1988) Oncogene jun encodes a sequence-specific
trans-activator similar to AP-1. Nature 332, 166-171.
Bechler, B., Cogoli, A. & Mesland, D. (1986) Lymphocyten
sind schwerkraft empfindlich. Naturwissenschaften 73, 400-403.
Busch, S.J. & Sassone-Corsi, P. (1990) Dimers, leucine
zippers and DNA-binding domains. TIG 6, 36-40.
Carpenter, G. (1987) Receptors for epidermal growth factor
and other polypeptide mitogens. Ann. Rev. Biochem. 56, 881-914.
Chirgwin, J.M., Przybyla, A.E., MacDonald, R.J. & Rutter,
W.J. (1979). Isolation of biologically active ribonucleic acid from sources
enriched in ribonuclease. Biochemistry 18, 5294-5299.
Cogoli, A., Tschopp, A. & Fuchs-Bislin, P. (1984) Cell
sensitivity to gravity. Science 225, 228-230.
Curran, T., Peters, C., Van Beveren, C., Teich, N. &
Verma, I.M. (1982). FBJ murine osteosarcoma virus: Identification and molecular
cloning of biologically active proviral DNA. J.of Virol. 44, 674-682.
de Groot, R.P., Rijken, P.J., Boonstra, J., Verkleij, A.J.,
de Laat, S.W. & Kruijer, W. (1990a) Epidermal growth factor induced expression
of c-fos is influenced by altered gravity conditions. Aviat. Space Environ.
Med., in press.
de Groot, R.P., Kruyt, F.A.E., van der Saag, P.T. &
Kruijer, W. (1990b) Ectopic expression of c-jun leads to differentiation of
P19 embryonal carcinoma cells. EMBO J. 9, 1831-1837.
de Groot, R.P. and Kruijer, W. (1990c) Transcriptional activation
by TGF.²1 mediated by the dyad symmetry element (DSE) and the TPA responsive
element (TRE). Biochem. Biophys. Res. Comm. 168, 1074-1081.
Duke, J.C. (1983) Suppression of morphogenesis in embryonic
mouse limbs exposed in vitro to excess gravity. Teratology 27, 427-436.
Fisch, T.M., Prywes, R. & Roeder, R.G. (1987) c-fos
sequences necessary for basal expression and induction by the epidermal growth
factor, 12-o-tetradecanoyl phorbol-13-acetate and the calcium ionophore. Mol.
Cell. Biol. 7, 3490-3502.
Fort, Ph., Marty, L., Piechaczyk, M., ElSabrouty, S., Dnai,
Ch., Jeanteur, Ph. & Blanchard, J.M. (1985). Various rat adult tissues express
only one major mRNA species from the glyceraldehyde-3-phophate-dehydrogenase
multigene family. Nucl.Acids Res. 13, 1431-1442.
Gilman, M.Z., Wilson, R.N, & Weinberg, R.A. (1986) Multiple
protein binding sites in the 5' flanking region regulate c-fos expression. Mol.
Cell. Biol. 6, 4305-4315.
Gilman, M.Z. (1988) The c-fos serum response element responds
to protein kinase C-dependent and -independent signals but not to cyclic AMP.
Genes & Dev. 2, 394-402.
Gorman, C.M., Moffat, C.P. & Howard, B.H. (1982). Recombinant genes
which express recombinant chloroamphenicol acetyltransferase in mammalian cells.
Mol.Cell.Biol. 2, 1044- 1051.
Greenberg, M.E., Siegfried, Z. & Ziff, E.B. (1987) Mutation
of the c-fos gene dyad symmetry element inhibits serum inducibility of transcription
in vivo and the nuclear regulatory factor binding in vitro. Mol. Cell. Biol.
7, 1217-1225.
Halstead, T.W. & Dutcher, F.R. (1987) Plants in space.
A. Rev. Pl. Physiol. 38, 317-345.
Henthorn, P., Zervos, P., Raducha, M., Harris, H. &
Kadesh, T. (1988). Expression of a human placenta alkaline phosphatase gene
in transfected cells: Use as a reporter for studies of gene expression. Proc.Natl.Acad.Sci.
U.S.A. 85, 6342-6346.
Imler, J-L. & Wasylyk, B. (1989) AP1, a composite transcription
factor implicated in abnormal growth control. Prog. in Growth Factor Res. 1,
69-77.
Kelly, K. & Siebenlist, U. (1986) The regulation and
expression of c-myc in normal and malignant cells. A. Rev. Immunol. 4, 317-338.
Kumei, Y., Nakajima, T., Sato, A., Kamata, N. & Enomoto,
S. (1989) Reduction of G1 phase duration and enhancement of c-myc gene expression
in HeLa cells at hypergravity. J. of Cell Science 93, 221-226.
Lorenzi, G., Fuchs-Bislin, P. & Cogoli, A. (1986) Effects
of hypergravity on whole-blood cultures of human lymphocytes. Aviat. Space Environ.
Med. 57, 1131-1135.
Melton, D.A., Krieg, P.A., Rebagliatie, M.R., Maniatis,
T., Zinn, K. & Green, M.R. (1984) Efficient in vitro synthesis of biologically
active RNA and RNA hybridization probes from plasmids containing a bacteriophage
SP-6 promoter. Nuc. Acids Res. 12, 7025-7056.
Müller, R. & Wagner, E.F. (1984) Differentiation
of F9 teratocarcinoma stem cells after transfer of c-fos proto-oncogenes. Nature
311, 438-442.
Prywes, R., Dutta, A., Cromlish, J.A. and Roeder, R.G. (1988)
Phosphorylation of serum response factor, a factor that binds to the serum response
element of the c-fos enhancer. Proc. Natl. Acad. Sci. USA 85, 7206-7210.
Rijken, P.J., de Groot, R.P., Briegleb, W., Kruijer, W.,
Verkleij, A.J., Boonstra, J. & de Laat, S.W. (1990) Epidermal growth factor
induced cell rounding is sensitive to simulated weightlessness. Aviat Space
Environ. Med., in the press.
Stumpo, D.J., Stewart, T.N., Gilman, M.Z. & Blackshear,
P.J. (1988) Identification of c-fos sequences involved in induction by insulin
and phorbol esters. J. Biol. Chem. 263, 1611-1614.
Suggs, S.V., Wallace, R.B., Hirose, T., Kawashima, E.H.
& Itakura, K. (1981) Use of synthetic oligonucleotides as hybridization
probes; Isolation of cloned cDNA sequences for human .²2-microglobulin. Proc.
Natl. Acad. Sci. USA 78, 6613-6617.
Tschopp, A. & Cogoli, A. (1983) Hypergravity promotes
cell proliferation. Experientia 39, 1323-1329.
Treisman, R. (1986) Identification of a protein binding
site that mediates transcriptional response of the c-fos gene to serum factors.
Cell 46, 567-574.
Treisman, R. (1987) Identification and purification of a
polypeptide that binds to the c-fos serum response element. EMBO J. 6, 2711-2717.
Ubbels, G.A. (12987) The role of gravity in the establishment
of the dors/ventral axis in the amphibian embryo. In: Sahm, P.R., Jansen, R.
& Keller, M.H. (eds.); Proceedings of the Nordermey Symp. on scientific
results of the german spacelab mission D1; Koln, Wissensch. projectfuhrung,
1987; Nordermey, Germany, august 1986.
Van Straaten, F., Müller, R., Curran, T., Van Beveren,
C. & Verma, I.M. (1983) Complete nucleotide sequence of a human c-onc: Deduced
amino acid sequence of the human c-fos protein. Proc. Natl. Acad. Sci. USA 80,
3183-3187.
Verma, I.M. & Sassone-Corsi, P. (1987). Proto-oncogene
fos: Complex but versatile regulation. Cell 51, 513-514.
FIGURE LEGENDS
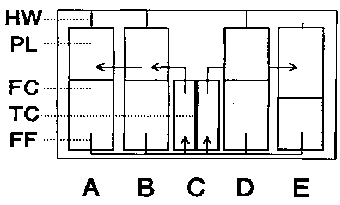
Figure 1 - Schematic representation of the CIS-1 plunger box experiment
unit.
The CIS-1 plungerbox experiment unit is a slight modification
of the unit used in the FROGS experiment flown on spacelab D1 (Ubbels, 1987)
and was designed and constructed by CCM (Nuenen, The Netherlands). A transverse
section of the module is shown. A431 cells are cultured on thermanox coverslips
(TC) and mounted in the central chamber (C). After activation of plunger (PL)
A by melting of a heater wire (HW), the cells are treated with EGF or medium
alone, which is present in the fluid compartment (FC). Washing of the cells
is performed by activation of plungers B and D, while the cells are lyzed by
activation of plunger E. Plunger E is shown in the activated position. The route
of the various solutions is indicated by arrows. After recovery, the lyzed cells
are collected from chamber C and RNA is isolated.
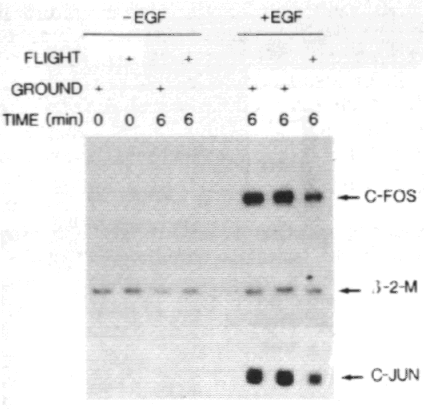
Figure 2 - Microgravity decreases EGF-induced c-fos and c-jun
expression.
A431 cells were cultured on thermanox coverslips, mounted in the CIS-1 plunger
box experiment units and assembled in the CIS-1 module on the MASER-3 sounding
rocket. As soon as microgravity was reached, the cells were treated with EGF
(+EGF, 6 min) or with medium alone (-EGF, 6 min). As a control one sample was
lyzed immediately after launch (-EGF, 0 min) to study the effects of the high
g-values reached during the launch of the rocket. Simoultaneously a 1-g reference
experiment (ground) was performed identically to the microgravity experiment
(flight). After all samples were washed and lyzed, RNA was isolated and analyzed
for c-fos, c-jun and .²-2-microglobulin transcripts by RNase
protection. Autoradiography was for 16 hours.
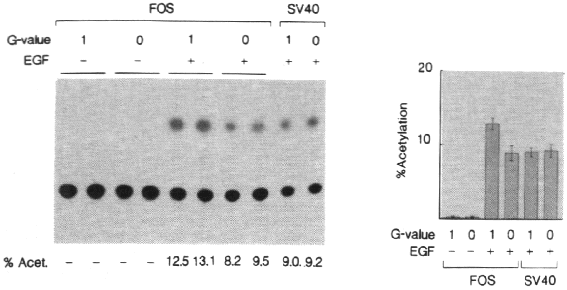
Figure 3 - Hypogravity decreases SRE activity.
A - A431 cells cultured in 75 cm2 flasks
were transfected with 10 µg of a construct containing three copies of
the c-fos serum response element (SRE, Treisman, 1986) coupled to the
HSV-thymidine kinase promoter and the bacterial CAT gene (FOS in the figure)
by Ca3(PO4)2 precipitation. Control cells were
transfected with an SV40 driven CAT gene (pSV2CAT, Gorman et al., 1982). After
24 hours the cells were trypsinyzed and replated on thermanox coverslips for
24 hours. The cells were mounted on a fast rotating clinostat and rotated for
2 hours at 60 rpm at 37oC. EGF (+EGF) or medium alone (-EGF) was
added and after rotation in the clinostat for 8 hours the cells were collected
and CAT activity was determined on TLC plates. 20 µg of cell extract was
routinely used for CAT assays (2µg for pSV2CAT transfected cells). Control
cells were incubated identically in non-rotating fixed clinostat tubes (1G in
figure).
B - The CAT activity of 4 independent experiments
as in A was quantified by liquid scintillation counting of 14C TLC
spots. Error bars indicate standard deviation.
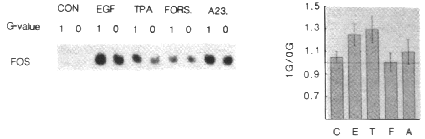
Figure 4 - Differential sensitivity of discrete signal transduction pathways
for hypogravity.
A - A431 cells were cultured on thermanox coverslips
for 48 hours before mounting on a fast rotating clinostat. Cells were rotated
at 60 rpm (0G in figure) or cultured in fixed tubes for 2 hours before addition
of EGF (100 ng/ml), TPA (100 ng/ml), forskolin (Fors, 10 µM), A23187 (A23,
2.5 µM) or medium alone (CON). After 15 minutes the cells were lyzed and
RNA was isolated. c-fos expression was determined by Northern blotting
as described in materials and methods. Autoradiography was for 24 hours. B
- The results of 3 independent experiments as in A were quantified by scanning
of the autoradiographs. Bars represent the ratio of c-fos expression
in control cells (1G) and rotated cells (0G). Error bars represent the standard
deviation.
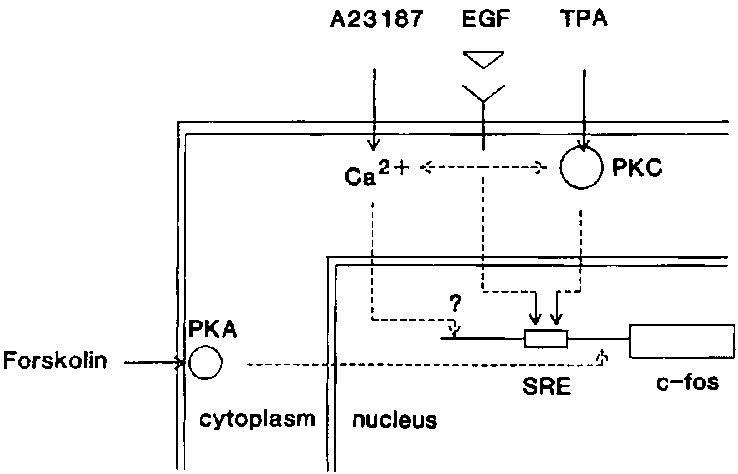
Figure 5 - Schematic representation of different signal transduction pathways
leading to enhanced c-fos expression.




