Development of Tissue Culture Techniques and
Hardware to Study Mineralization under Microgravity Conditions.
J.J.W.A. van Loon1, J.P. Veldhuijzen1,
E.J. Windgassen2, T. Brouwer3, K. Wattel3,
M. van Vilsteren3 and P. Maas4.
Academic Centre for Dentistry Amsterdam
(ACTA), Dept. of Oral Cell Biology1 and Dept. of Engineering2
and Vrije Universiteit, Fac. of Biology, Dept. of Electronics3, and
of Engineering4. Amsterdam, The Netherlands.
ABSTRACT
To study the effects of weightlessness on
fetal mouse long bone rudiment growth and mineralization we have developed a
tissue culture system for the Biorack facility of Spacelab. The technique uses
standard liquid tissue culture medium, supplemented with Na-b-glycerophosphate,
confined in gas permeable polyethylene bags mounted inside ESA Biorack Type-I
experiment containers. The containers can be flushed with an air/5% CO2
gas mixture necessary for the physiological bicarbonate buffer used. Small amounts
of fluid can be introduced at the beginning (e.g. radioactive labels for incorporation
studies) or at the end of the experiment (fixatives). A certain form of mechanical
stimulation (continuous compression) can be used to counteract the, possibly,
adverse effect of microgravity. Using 16 day old metatarsals the in vitro
calcification process under microgravity conditions can be studied for a 4 day
period.
Key words: bone, weightlessness,
in vitro, mineralization, mechanical stimulation, bicarbonate buffer,
spacelab.
Published in: Advances in Space Research,
14(8), 289-298, 1994.
2.1 INTRODUCTION
It is well known that the mechanical environment
is an important factor in the development and maintenance of skeletal tissues.
Increased mechanical stress on bone tissue changes the bone density and morphology,
resulting in an increased bone mass.(2) A low mechanical stress environment
leads to a rapid bone loss as is demonstrated in immobilization(15)
and bedrest(11) studies. In various experimental set-ups such as
tail suspension(1,5) or under actual microgravity(12)
during space flight, a decrease in bone mass is reported which is related to
a decreased mineral deposition.
There is a very limited number of reports
on a change in direct effects of osteoclastic bone resorption under actual microgravity
conditions.(4,6,7,19) Whether the effects on bone cells in vivo
are indeed caused directly by a changed mechanical stress regime or that the
response of bone is the result of a changed amount of circulating hormones which
act on bone cell metabolism (such as corticosteroids or 24,25(OH)2D),
is not clear.(13)
To exclude effects of systemic factors,
we are using an in vitro model to study the effect of mechanical loading
on skeletal tissues. We have shown that intermittent (IC) and continuous compression
(CC) of 13 kPa above ambient increases mineralization in 16 days old fetal mouse
long bone rudiments and calvaria,(8,10) whereas in 17 day old metatarsals
and calvaria resorption of the mineralized matrix by osteoclasts was decreased.(3,8)
It is hypothesized that non-compressed control bones, which show a decreased
mineralization and an increased resorption, resemble a situation of disuse.
To examine if microgravity is an even more extreme situation of disuse it is
tempting to study the reaction of these fetal metatarsal long bone rudiments
cultured under microgravity conditions with and without compression. In order
to conduct such an experiment in the Biorack facility of Spacelab
we have developed a culture system and the appropriate hardware to be used in
the standard Biorack Type-I containers. This experiment, 02-NL Bones, is planned
to fly on board the Space Shuttle during the first International Microgravity
Laboratory (IML-1) mission, STS-42. This paper describes the organ culture techniques
and hardware developed for an in vitro study with fetal mouse long bone
rudiments under microgravity with or without compression. The same techniques
seem also applicable for in vitro studies of other mammalian organs,
tissues and cells under microgravity.
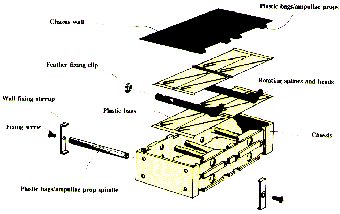
Fig. 2.1. Opened Type-I/E container (20´40´80 mm) with experiment hardware fitted
to the lid. a: one way valve for introducing the gas mixture, b: holes for ample
flushing the gas mixture between the culture bags, c: connector to Biorack power
and data lines.
2.2 MATERIALS AND METHODS
2.2.1 Long Bone Rudiments
16 Day old foetuses of Swiss albino mice
were used. The metatarsals (average dimensions: length 1.2 mm, diameter 0.4
mm) are carefully dissected and cleared from adhering tissues without disturbing
the perichondrium and rinsed in Puck's buffer G (Gibco, Paisley, Scotland).
Fig. 2.2. Details of experiment
hardware inside a Type-I/E container. a: pressure sensor, b: glass ampoule,
c: metal spline.
2.2.2 Standard Laboratory Procedures
Rudiments are cultured up to 4 or 5 days
in standard 24 well tissue culture plates (Greiner) in 300 ml of aMEM medium
without nucleosides (Gibco), supplemented with 10% heat inactivated normal rat
serum (TNO, The Netherlands) and 100 units/ml penicillin/streptomycin (Difco).
To sustain good mineralization an organic phosphate source, 0-2.0 mM Na-b-glycerophosphate
(Sigma) is used.(16,17) Control rudiments are cultured in a standard
air/5% CO2 incubator (37°C, 100% humidity).
Rudiments cultured under continuous compression
(CC) are placed in a stainless steel culture chamber (37°C, air/5% CO2,100%
humidity) in which the gas phase is compressed at 13 kPa above ambient. Rudiments
cultured under CC are exposed in vitro to a hydrostatic compression in
the same order of magnitude as the calculated muscle force acting upon these
rudiments in vivo.(10)
The 16 day old rudiments, which are not
calcified at the beginning of the experiment, calcify during the culture period.
Determination of the length of the calcified zone (diaphysis) is used as a measure
for the mineralization process.
2.2.3 Hardware and Procedures
The experiments in Biorack will be performed
in Type-I containers (dimensions: 2´4´8 cm, Fig. 2.1). We use a modified version
of the hardware described by Richoilley and Tixador.(14,18) Basically,
the rudiments are cultured in bags composed of two layers of gas permeable polyethylene
(40´20´2 mm).
The bags are filled with 670 ml culture
medium (same components as in standard laboratory conditions) and include a
glass ampoule containing 25 ml liquid: e.g. radioactive labels or fixative (Fig.
2.2). In the long side of the containers a small stainless steel one-way valve
is mounted in order to introduce a gas mixture of air/5% CO2 for
flushing and pressurization (Fig. 2.1a). Containers can accommodate 16 culture
bags, each bag containing one long bone rudiment.
Fig. 2.3. Major parts and connections
of the hardware for the Biorack experiment Bones-02NL. a: Type-I/E container,
b: syringe for pressurization, c: PROD, d: pressure bottle with needle valve
containing air/5%CO2, e: connection to the Biorack power and data
lines.
To release the content of the ampoule into
the culture medium they are broken, by the astronauts, at a predetermined place
by turning a spline situated between the culture bags (Fig. 2.2b, 2.2c). In
the sides and the cover plates of the hardware, holes are made to provide ample
gas flow during flushing (Fig. 2.1b).
The gas mixture of air/5% CO2
is contained in a 150 ml stainless steel pressure bottle with needle valve (Whitey,
Ohio) pressurized at 8.0×106 Pa (80 bar) (Fig. 2.3d). This volume
is sufficient to serve an experiment of 4 days in which eight containers are
flushed and pressurized 2 times. In containers which are pressurized (CC= continuous
compression; 113 kPa) a volume equivalent to 4 culture bags is devoted to accommodate
newly developed electronics. These include a pressure sensor (Druck Ltd., UK;
Fig. 2.2a) and electronics to correct for the temperature difference during
pressurization (which is performed outside the incubator at room temperature
in the glove box) and during culture in the incubator (36°C). A special Pressure
Read-Out Device (PROD) is developed to monitor the increase in pressure indicated
by colored LED's (Fig. 2.3c).
Pressurization is performed by pushing down
the syringe piston (Fig. 2.3b). During pressurization the PROD is connected
to the power and data lines of Biorack and to the container (Fig. 2.3e, 2.3a).
During pressurization at room temperature a green LED is activated when the
actual pressure is such that the final pressure, when placing the containers
into the 36°C incubator, will be 113 ± 0.5 kPa. The actual pressure read-out
via the Biorack data lines will be with an accuracy of 1 Pa.
Since both flushing and pressurization of
the containers and breaking the ampoule has to be performed by the astronauts,
this technique is only applicable for manned spaceflights.
2.2.4 Statistics
Results are given as mean ± standard error
of the mean (SEM); significance is calculated using Student-t test statistics,
5% two-sided probability.
2.3 RESULTS
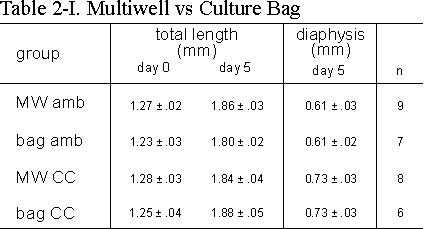
Table 2.I: The effect of growth (total length) and length of calcified zone
(diaphysis) of 16 day old metatarsals cultured in standard tissue culture wells
(MW) or polyethylene bags (bag) with (CC) or without (amb) continuous compression
of 13 kPa above ambient. Media supplemented with 2.0 mM bGP. Values are mean
± SEM, *p £ 0.05.
2.3.1 Culture Bags Versus Multiwells
Since we use in our system a fluid culture
medium, special precautions have to be taken.
In a series of experiments we have compared
the growth and mineralization of the long bones cultured in polyethylene culture
bags and in multiwells both under ambient and CC conditions (Table 2.I). It
is clear from these data that the growth of the rudiments (total length at the
end of the 5 day culture period) in culture bags compared to multiwells is not
significantly different. CC did not affect the increase in total length in the
two culture conditions investigated. We see however a significant increase in
length of the mineralized diaphysis under CC conditions both in multiwells as
well as in culture bags.
2.3.2 Effect of Temperature
One of the advantages in the Space Shuttle/Spacelab
experiment time line is the possibility of a relatively late access for biological
experiments. However, there is always a period of about 24 hours between the
handover of the samples and the actual start of the experiment in Spacelab's
Biorack while in orbit.
We have therefore tried to minimize biological
activity during this period by keeping the experiment containers at a lower
temperature than in the normal culture procedure.
Fig. 2.4 shows the effect on growth (A)
and mineralization (B) after a period of 24 hours at 22°C followed by a transfer
to the normal culture temperature of 37°C for 5 days. Fig. 2.4A shows that after
24 hours at 22°C, the length of the rudiment is not significantly changed and
is still comparable with the starting value (day 0) of the 37°C group. Also
after 4 or 5 days of culture at 37°C there is no significant difference in growth
between the two groups. Fig. 2.4B shows that mineralization is arrested for
24 hours. The metatarsals started to calcify in the first 24 hours at 37°C,
whether the culture started directly at 37°C or after a 24 hour preculture period
at 22°C. Also, the length of the mineralized diaphyses of the two groups, when
measured after 4 or 5 days of culture at 37°C, is not significantly different.
No differences were found between a culture
temperature of 36°C (Biorack incubator C) and the standard 37°C (data not shown).
2.3.3 Effect of Na-b-glycerophosphate
Fig. 2.5 shows the effect of the addition
of various concentrations of Na-b-glycerophosphate (bGP) to the culture medium.
The length of the mineralized diaphyses of 16 day old rudiments (cultured in
multiwells) was measured after a culture period of 5 days. It is clear that
without bGP no calcification occurred. The maximum effect of bGP is reached
at a concentration of 1.0 mM. Increasing the concentration up to 2.0 mM did
not change the effect on mineralization.
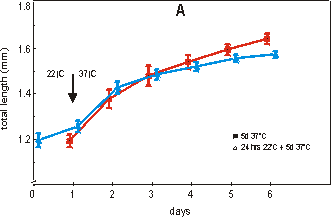
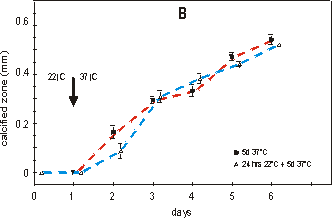
Fig. 2.4. Effect of growth: total length (A) and mineralization: length of calcified
zone (B) of 16 day old metatarsals directly cultured at 37°C (filled square)
or with a 24 hour lag period of 22°C (open triangle). Media supplemented with
2.0 mM of bGP. Values are mean ± SEM, n=7.
2.3.4 Effect of Gas Phase
In all experiments a physiological sodiumbicarbonate
buffer is used, which is the most common type of buffer applied in tissue culture.
The use of this buffer requires a special gas mixture of air/5% CO2.
This poses some additional difficulties in the hardware development for Biorack
experiments. Other buffers which do not require a special gas mixture are available
but results from pilot experiments (data not shown) with HEPES as buffer (which
requires only air as gas phase) show abnormal growth and mineralization, especially
under CC. In the former experiments culture bags are placed in a 5% CO2-incubator.
However, in the Biorack experiment the culture bags will be placed in a closed
Type-I containers. The remaining air volume in a filled container is relatively
small and has to be flushed with the air/5% CO2 gas mixture. We have
compared the growth and mineralization of long bone rudiments cultured in polyethylene
bags placed in an incubator or in bags mounted in a Biorack Type-I container.
In a container the maximum number of 16 culture bags were placed. For the 4
day culture period containers were flushed at the beginning of the experiment
and after 2 days (Fig. 2.6b), or only at the beginning of the culture (Fig.
2.6c). Long bones cultured in a standard multiwell placed in a CO2-incubator
were used as controls (Fig. 2.6a).
Both the increase in total length and the
length of the mineralized diaphysis are less in containers closed for 4 days,
compared to metatarsals cultured in the incubator. Cultures flushed after 2
days did not differ from the control cultures both in increase in total length
and mineralization when measured at the end of the 4 day culture period.
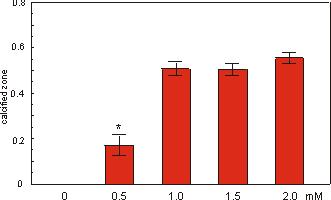
Fig. 2.5. Effect of Na-b-glycerophosphate on the mineralization (expressed in
length of calcified zone) of 16 day old mouse long bone rudiments cultured for
5 days. Values are mean ± SEM, *p £ 0.05 compared to 1.0 mM bGP, n=7.
2.4 DISCUSSION
We have adopted the use of double layered
polyethylene culture bags for this experiment.(14,18) When the medium
is preconditioned with 5% CO2 in an incubator, filling the bags with
medium, ampoule, long bone rudiment and the subsequent sealing of the bag does
not change the pH of the medium seriously.
When we consider growth (total length) of
the rudiments it is clear from experiments shown in Table 2.I that cultures
of long bone rudiments in polyethylene bags are very good comparable with cultures
in conventional multiwells. Also, mineralization in culture bags (placed in
a 5% CO2-incubator) and in multiwells did not differ significantly.
Applying continuous compression as a model for mechanical stimulation resulted
in an increase in mineralization both in polyethylene bags and in standard multiwells
(Table 2.I).
Also 17 day old fetal mouse long bone rudiments
respond to mechanical forces.(3,9) 17 Day old metatarsals have the
same growth characteristics compared to 16 day old. Therefore we assume that
17 day old bones can be used as a resorption model in the same hardware.
We used continuous compression instead of
intermittent compression, although the latter represents a more physiological
condition and yields a higher response.(10) However, to accommodate
a pulsating device inside the relatively small Type-I container would leave
very little free space and thus reducing the number of samples.
In the time line of microgravity experiments
there is always a lag period between the handover of the experiment to the launch
authorities and the activation of the experiment in orbit. Fortunately in Spacelab
missions aboard the Space Shuttle this lag period is rather short. Latest handover
is about 14 hours before launch, while Biorack is operational in about 10 hours
after launch. Under tissue culture conditions there is the possibility to reduce
biological activity during this lag period by reducing the amount of serum in
the culture medium. In our experimental set-up this would mean that serum has
to be included into the glass ampoule. Breaking of the glass ampoule should
introduce serum into the medium and activate cell metabolism. However, tests
to prepare glass ampoules containing serum showed that serum interfered unpredictable
with the sealing procedure causing frequent leakages. Therefore we have included
serum into the culture medium; the ampoules contain only the radioactive labels
or fixatives. Lowering the temperature to 22°C, which is the ambient temperature
of the Shuttle middeck stowage compartment, arrests the biological activity
of the long bone rudiments during this lag period (Fig. 2.4). A culture period
of 4 days (at 37°C), whether or not preceded by a 24 hour period of 22°C, resulted
in the same increase in both the total length of the rudiment (Fig. 2.4A) and
mineralization (Fig. 2.4B). Thus beginning with mounting of the culture bags
in the containers, during handover, experiment integration and stowage, the
containers must be kept at ambient temperatures (22°C). After activation of
Biorack (in orbit) the containers are placed by the astronauts in the 36°C incubator
where the tissues metabolism reaches normal in vitro levels rapidly.
By breaking the ampoule with radioactive labels the incorporation of label can
start only when under microgravity conditions.
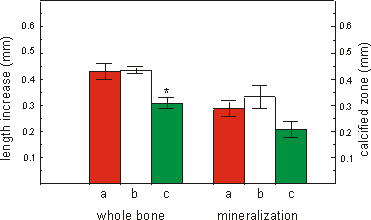
Fig. 2.6. The effect of flushing a Type-I container with air/5% CO2
mixture on growth (increase in total length) or mineralization (length of calcified
zone) of 16 day old mouse metatarsals cultured for 4 days. a: control cultures
in normal tissue culture plates in a CO2 incubator (n=5). b: culture
in polyethylene bags inside a Type-I container flushed at day 0 and after 2
days with air/5% CO2 (n=5). c: as b but flushed only at day 0 and
kept closed for the 4 day culture period (n=6). Media are supplemented with
1.0 mM bGP. Values are mean ± SEM, *p £ 0.05.
Our data show that 16 day old mouse long
bone rudiments calcify readily during a 4 day culture period, provided that
Na-b-glycerophosphate (1.0-2.0 mM) is added to the medium.
In multiwells long bone rudiments adhere
with fibroblastic outgrowth to the bottom of the well which minimizes curvation
of the rudiment during growth. However in culture bags adherence does not occur.
We noted that to prevent curvation during growth not less than 1.0 mM Na-b-glycerophosphate
should be added. We have no explanation for this phenomenon.
In culturing long bones in polyethylene
bags placed into a Type-I container only a limited amount of gas mixture is
present (about 2.0-2.5 ml gas mixture per 0.670 ml medium). Experimental conditions
comparable with cultures in standard multiwells in a CO2 incubator
are met, providing the gas mixture is changed after 2 days (Fig. 2.6).
Because of limited stowage place available
for Biorack, only a relative small pressure bottle can be accommodated. Therefor
a total culture period of 4 days is chosen.
At the end of the experiment the samples
for histological evaluations will be fixed and stored in the on board cooler,
samples used for labeling studies (45Ca, 32P incorporation)
will be stored in the on board freezer (-10°C).
We conclude that polyethylene culture bags,
in combination with the Na-bicarbonate buffered aMEM medium supplemented with
1.0 mM of bGP and flushing of the experiment containers after 2 days with an
air/5% CO2 gas mixture, are very suitable to culture 16 day old long
bone rudiments in Biorack Type-I containers. Under these culture conditions
also the effect of compression can be studied in modified Type-I/E containers
which are pressurized using the gas mixture from the pressure bottle. With the
hardware used pressures can be monitored very precisely; PROD ± 0.5 kPa; Biorack
data-lines ± 1 Pa.
With two different aged fetal mouse long
bones (16 and 17 day old) as in vitro models it should be possible to
determine the effect of microgravity on growth, mineralization and resorption
in skeletal tissues without the interference of systemic factors occurring in
vivo.
Since the culture method is based on conventional
tissue culture techniques using a bicarbonate buffered medium, this hardware
might also be used for the study of other tissues, organs, and cells under microgravity
conditions.
2.5 REFERENCES
1 Abram A.C., Keller T., Sprengler
M. The effects of simulated weightlessness on bone biomechanical and biochemical
properties in the maturing rat. J. Biomechanics 21(9), 755-767, 1988.
2 Block J.E., Friedlander A.L., Brooks
G.A., Steiger P., Stubbs H.A., Genant H.K. Determinants of bone density among
athletes engaged in weight-bearing and non-weight-bearing activity. J. Appl.
Physiol. 67(3), 1100-1105, 1989.
3 Burger E.H., Veldhuijzen J.P.,
Klein Nulend J., Van Loon J.J.W.A. Osteoclastic invasion and mineral resorption
of the fetal mouse long bone rudiments are inhibited by culture under intermittent
compressive force. Conn. Tissue Res. 120, 131-141, 1989.
4 Cann C.E., Adachi R.R. Bone resorption
and mineral excretion in rats during spaceflight. Am. J. Physiol. 244, R327-R331,
1983.
5 Globus R.K. , Bikle D.D., Morey-Holton
E. Effects of simulated weightlessness on bone mineral metabolism. Endocrinology
114(6), 2264-2270, 1984.
6 Földes I., Rapcsák
M., Szilágyi T., Organov V.S. Effects of space flight on bone formation
and resorption. Acta Physiologica Hungarica. 75(4), 271-285, 1990.
7 Kaplanski A.S., Durnova G.N., Ilyina-Kakueva
E.I. Histomorphometric analysis of bone of Cosmos-1887 rats. Physiologist. 33(1),
s20-s30, 1990.
8 Klein-Nulend J., Veldhuijzen J.P.,
de Jong M., Burger E.H., Increased bone formation and decreased bone resorption
in fetal mouse calvaria as a result of intermittent compressive force in vitro.
Bone and Min. 2, 441-448, 1987.
9 Klein-Nulend J., Veldhuijzen J.P.,
Van Strien M.E., de Jong M., Burger E.H. Inhibition of osteoclastic bone resorption
by mechanical stimulation in vitro. Arthritis Rheum. 33(1), 66-72, 1990.
10 Klein-Nulend J., Veldhuijzen J.P.,
Burger E.H. Increased calcification of growth plate cartilage as a result of
compressive force in vitro. Arthritis Rheum. 29(8), 1002-1009, 1986.
11 LeBlanc A.D., Schneider V.S.,
Evans H.J., Engelbretson D.A., Krebs J.M. Bone mineral loss and recovery after
17 weeks of bed rest. J. Bone and Min. Res. 5(8), 843-850, 1990.
12 Morey E.R., Baylink D.J. Inhibition
of bone formation during space flight. Science 201(4361), 1138-1141, 1978.
13 Morey-Holton E.R., Schnoes H.K.,
DeLuca H.F., Phelps M.E., Klein R.F., Nissenson R.H., Arnaud C.D. Vitamin D
metabolites and bioactive parathyroid hormone levels during Spacelab 2. Aviat.
Space Environ. Med. 59: 1038-1041, 1988.
14 Richoilley G., Tixador R., Gasset
G., Templier J., Planel H., Preliminary results of the Paramecium experiment.
Naturwissenschaften 73, 404-406, 1986.
15 Rodríguez J.I, Palacios
J., García-Alix A., Pastor I., R.Paniagua R. Effects of immobilization
on fetal bone development. A morphometric study in newborns with congenital
neuromuscular diseases with intrauterine onset. Calcif. Tissue Int. 43, 335-339
1988.
16 Tenenbaum H.C. Role of organic
phosphate in mineralization of bone in vitro. J. Dent. Res. 60(C), 1586-1589,
1981.
17 Tenenbaum H.C., McCulloch C.A.G.,
Fair C., Birek C. The regulatory effect of phosphates on bone metabolism in
vitro. Calcif. Tissue Int. 257, 555-563, 1989.
18 Tixador R., Richoilley G., Gasset
G., Moatti N., Lapchine L., Raffin J., Zaloguev S., Kojarinov V.I., Lepskye
A.A., Appareillage developpe pour la determination "in vitro" de la C.M.I. pendant
un vol spacial. Innov. Tech. Biol. Med. 4(4),401-406, 1983.
19 Vico L., Chappard D., Alexandre
C., Palle S., Minaire P., Riffat G., Novicov V.E., Bakulin A.V. Effects of weightlessness
on bone mass and osteoclast number in pregnant rats after a five-day spaceflight
(Cosmos 1514). Bone. 2, 95-103, 1987.
2.6 ACKNOWLEDGMENTS
This study is supported by grant MG-004
from the Space Research Organization of the Netherlands (SRON).





