Decreased Mineralization and Increased Calcium Release
in Isolated Fetal Mouse Long Bones under Near Weightlessness.
Jack J.W.A. van Loon, Dirk-Jan Bervoets,
Elisabeth H. Burger, Suzanne C. Dieudonné, Jan-Willem Hagen, Cor M. Semeins,
Behrouz Zandieh Doulabi, J. Paul Veldhuijzen
Academic Centre for Dentistry Amsterdam
(ACTA), Dept. of Oral Cell Biology, Amsterdam, The Netherlands.
ABSTRACT
Mechanical loading plays an important role
in the development and maintenance of skeletal tissues. Sub-normal mechanical
stress as a result of bed rest, immobilization, but also in spaceflight, results
in a decreased bone mass and disuse osteoporosis, whereas supra-normal loads
upon extremities result in an increased bone mass.
In this first in vitro experiment
with complete fetal mouse cartilaginous long bones, cultured under microgravity
conditions, we studied growth, glucose utilization, collagen synthesis, and
mineral metabolism, during a 4 day culture period in space. There was no change
in %length increase and collagen synthesis under microgravity compared to in-flight
1gravity. Glucose utilization and mineralization were decreased under
microgravity. In addition mineral resorption, as measured by 45calcium
release, was increased.
These data suggest that weightlessness has
modulating effects on skeletal tissue cells. Loss of bone during spaceflight
could be the result of both impaired mineralization as well as increased resorption.
Key words: bone, microgravity,
mineralization, resorption, mechanical loading.
Accepted for publication in the Journal
of Bone and Mineral Research, April, 1995.
3.1 INTRODUCTION
It has been well documented that bone is
sensitive to its mechanical environment. A unique condition of skeletal unloading
occurs during spaceflight, where the 1gravity weightbearing stimulation
of the skeleton is absent. From one of the first spaceflight studies with humans(1,2)
and rats(3,4) it has become clear that osteoblastic bone formation
is retarded compared to Earth controls. Most spaceflight studies show no effect
with respect to bone resorption by osteoclasts,(3,4) but some have
indicated an increased resorption activity under near weightlessness conditions
in vivo, although the various recovery times, as in most spaceflight
studies, must be kept in mind.(5-8) It is not clear, however, whether
these results on bone metabolism in vivo are a result of the lack of
weightbearing, which acts locally, or whether systemic factors are also involved.
Previous experiments have shown that isolated
fetal mouse metatarsal long bones respond to changes in mechanical loading.
An increase of mineralization has been reported when these metatarsals were
cultured under intermittent hydrostatic pressure(9) or in a hypergravity
field.(10) In addition, mineral resorption was decreased in metatarsals
cultured under intermittent compression for four days.(11)
In the present study we used similar organ
cultures to study growth, glucose utilization, collagen synthesis and mineral
metabolism under spaceflight conditions during a 4 day culture period in microgravity.
Using these parameters the hypothesis was tested that weightlessness influences
the metabolic activity of skeletal tissues in vitro.
This microgravity experiment was performed
during the first International Microgravity Laboratory mission (IML-1) of the
Space Shuttle program in the Biorack facility of SpaceLab.
Fig. 3.1 ED16 mouse metatarsal
long bones. A: immediately after dissection B: cultured under microgravity conditions
C: cultured in the in-flight 1g centrifuge. Bar= 0.5 mm. During
the 4 day culture period of an ED16 fetal mouse long bone, mineralization starts
in the ossifying center of the rudiment (diaphysis). The mineralized diaphysis
is clearly visible as an opaque part in the center of the rudiment (B and C).
TL is total length of the metatarsal, D is length of the diaphysis.
3.2 MATERIALS AND METHODS
3.2.1 Biorack
This experiment was performed in the Biorack
facility of SpaceLab. Biorack is a multi user facility and consists of incubators,
a cooler, a freezer and a sealed workbench, the glovebox.(12) Two
identical Biorack models were used; one was flown in SpaceLab (Flight Model),
the other remained on Earth (Ground Model) as reference.
Biorack has an important advantage over
other microgravity experiment facilities in that it provides a small radius
(78 mm), slow rotating (107.0±0.5 rpm) centrifuge. This centrifugal force resulted
for the Flight Model in a 1g acceleration, generating the unit gravity
as present on Earth. Due to the geometry of the centrifuge and the location
of samples this g-level ranges from 0.890 to 1.151g. The biological
material of the microgravity group as well as the in-flight 1g group
are both exposed to the same launch vibrations, accelerations, cosmic radiation
or other environmental conditions. The only difference between the two flight
groups is the gravity parameter. Centrifugation in the Ground Model results
in a 2g (1.338-1.525g) load; the resultant of 1g
from the Earth and 1g from the centrifuge. Also other experiments were
accommodated on this 1g centrifuge. Due to handling of other experiments
the centrifuge was stopped 10 times during the four day culture period. Each
stop lasted no longer then 1.5 minutes. The g-vector acting on the metatarsals
was oriented perpendicular to the rudiments long axis.
Fig. 3.2. ED17.5 mouse metatarsal
long bones. A: immediately after dissection B: cultured under microgravity conditions
C: cultured in the in-flight 1g centrifuge. Bar= 0.5 mm.
3.2.2 Tissue preparation / culture procedure
Isolated embryonic mouse cartilaginous long
bones (metatarsals) were cultured for 4 days under near weightlessness, or control
conditions. The culture procedure and hardware are described in detail elsewhere.(13)
Briefly, per fetus the middle 3 metatarsals in each foot of 16 days embryonic
(ED16) (Fig. 3.1) and 17.5 days embryonic (ED17.5) (Fig. 3.2) Swiss random mice
(Harlan Sprague-Dawley, Indianapolis) were used. ED16 metatarsals had not yet
calcified and start to calcify during culture (Fig. 3.1). ED17.5 rudiments had
calcified in utero and start to resorb during culture (Fig. 3.2). Metatarsals
were aseptically harvested and individually cultured in gas permeable polyethylene
bags. The experiment was accommodated in eight small standard Biorack Type-I
containers (204080 mm),(12) inside Biorack; four
flight and four ground containers. Each container held 16 bags. Each bag contained
one metatarsal in 670 l culture medium and one 25 ml glass ampoule.
For metatarsals used for biochemical studies this ampoule was filled with radioactive
labels. For the ED16 biochemical samples, ampoules contained 45Ca,
32P and 3H-Pro. Ampoules used for the ED17.5 samples contained
only 3H-Pro. The ampoules for an additional set of containers, used
for histological evaluations, contained only formaldehyde.
The metatarsals were kept in a metabolically
inactive state during a 24 hour lag period,(13) used for transportation
and launch, by storage at room temperature (see timeline for more detail). When
the spacecraft was in orbit, all containers were transferred from stowage in
the middeck locker inserts to the Biorack glovebox. For the ED16 and ED17.5
biochemical samples, the ampoules were broken by the facility operators at the
beginning of the final 4 days of culture, to release the radioactive labels
into the medium. For the histological samples the ampoules, containing fixative,
were broken at the end of the experiment. Still in the glovebox, all containers
were flushed with a 5% CO2/in air gas mixture. They were then placed
in a 36°C incubator which initiated cell activity. After 2 hours, pressure increase
built up in the containers due to the temperature increase, was released. For
logistic reasons all ground operations lagged two hours behind the flight experiment.
3.2.3 Timeline
L = launch of STS-42
L-60 hrs: Mice for calcium release
studies were injected with 100 Ci 45Ca at 16.5 day
of gestation.
L-40 hrs: ED17.5 metatarsals
were dissected, photographed and pre-cultured o/n in a standard 5% CO2/in
air, 37°C, incubator.
L-27 hrs: ED16 metatarsals were
dissected, photographed, sealed into the tissue culture bags, and left
in a 5% CO2/in air incubator.
L-24 hrs: ED17.5 metatarsals
were rinsed, in fresh medium sealed into the tissue culture bags and
left in a 5% CO2/in air incubator.
L-20 hrs: ED16 and ED17.5 samples
were integrated into the Type-I containers. All containers were flushed
with 5% CO2/in air and kept at room temperature.
L-17 hrs: Hand-over of the experiment
containers to ESA officials, for inspection, and later transport to
the launch site at room temperature, in foam isolated middeck locker
inserts.
L=0 hrs: Launch of STS-42 (IML-1),
Jan. 22, 1992, 09:52 h EST.
L+5 hrs: Activation of Biorack.
Power-up of incubators and coolers to reach steady state before starting
the actual experiments.
L+6.5 hrs: Start of experiment,
by moving containers from the middeck lockers into the Biorack glovebox
to flush all containers and activate the samples (introducing the radio
isotopes) for biochemical studies. Subsequently all containers were
placed in the 36°C incubator, the microgravity samples in the static
racks, the in-flight 1g samples on the centrifuge. ('Day 0'
of the experiment).
L+57 hrs: All containers were
flushed again with 5% CO2/in air.
L+105 hrs: At day 4 the experiment
was terminated by placing the containers for biochemical analysis in
the -10°C freezer. The samples for histological evaluations were first
fixed, and then stored in the +4°C cooler.
L+8 days: Recovery of STS-42,
at Edwards Air Force Base (CA), at Jan. 30, 11:07 h (EST).
Containers were transported in 4°C and -10°C
Passive Thermal Conditioning Units (PTCU) from California to Kennedy Space Center
(KSC), and again from KSC to our laboratory in Amsterdam, the Netherlands.
3.2.4 ED16 metatarsals
Medium used for ED16 cultures consisted
of bicarbonate buffered MEM without nucleosides supplemented with 50
mg/l gentamicin and 0.5% v/v fungizone (Gibco), 1% heat inactivated rat serum
(TNO, The Netherlands), 50 mg/l L-ascorbic acid, 300 mg/l Glutamine (Merck)
and 1.0 mM Na--glycerophosphate (Sigma). The 16 day old metatarsals
also received, 1 Ci/ml 45Ca (specific activity; 1 Ci/mmol,
with an average of 6.69105 cpm per culture bag), 1 Ci/ml
32P (carrier free, with an average of 1.18106 cpm
per culture bag) and 1 Ci 3H-proline/ml (24 Ci/mmole) (Amersham)
by means of breaking the glass ampoule at the start of the final culture period
(Day 0). 45Ca and 32P were added to the medium to be incorporated
into the calcifying diaphysis of ED16 metatarsals only during the final 4 day
culture period (Fig. 3.1B and 3.1C). This part of the experiment was stopped
by placing the samples in a -10°C freezer until processing (see timeline). Finally,
mineral was extracted, using 10% TCA, and radioactivity in the TCA fraction
as well as in the medium was determined by liquid scintillation counting.
Other ED16 metatarsals were cultured without
radioactive labels, for histological evaluations (see below).
3.2.5 ED17.5 metatarsals
To study mineral resorption ED17.5 metatarsals
were used. Pregnant mice were injected intraperitoneally at day 16.5 of gestation
with 100 Ci 45CaCl2, to label the rapid mineralizing
bones of the embryos. One day later, 45Ca prelabeled metatarsals
were dissected and precultured at 37°C for one day to remove the freely exchangeable
45Ca. Subsequent culture conditions were identical to ED16 rudiments
but no 45Ca, 32P and Na--glycerophosphate were
added. The absence of Na--glycerophosphate prevented additional growth
of the mineralized diaphysis (Fig. 3.2). The glass ampoule only contained 3H-proline
at a final concentration of 1 Ci 3H-proline/ml. This part
of the experiment was stopped by placing the samples in a -10°C freezer until
processing. Percentage release of calcium was calculated by counting mobilized
45Ca in the culture medium, and measuring the remaining 45Ca
in the metatarsals after extraction with 10% TCA. Radioactivity was measured
using liquid scintillation counting.
Other ED17.5 metatarsals were cultured without
radioactive labels, for histological evaluations (see below).
3.2.6 Lengths
Data on lengths were assessed from photomicrographs
taken with an inverted microscope directly after dissection of the metatarsals,
and again after return of the samples. Total length and length of the mineralized
zone were measured directly from photographic negatives, after enlarging the
image using a professional enlarger. The lengths were measured with a standard
ruler with a one millimeter accuracy. The final magnifications were 177
and 145 for ED16 samples, and 177 and 91 for ED17.5
samples, for start and end point of the culture, respectively. Lengths are also
indicated in Fig. 3.1.
3.2.7 Glucose utilization
For glucose utilization, the remaining D-glucose
in the medium per metatarsal after culture was measured enzymatically. The NADPH
end-product was measured spectrophotometrically at 366 nm (Boehringer-Mannheim,
kit #716251). Fresh medium contained 1015 mg/l D-glucose.
3.2.8 Collagen synthesis
Relative collagen synthesis was assayed
according to Peterkofsky and Diegelmann, and Kream et al.(14,15)
The collagen values were corrected for the relative abundance of proline in
collagen compared to noncollagen proteins.(16) Specificity of the
batch collagenase (Worthington, New Jersey) was tested by digestion of 3H-labeled
non collagenous protein of tooth enamel (amelogenins, courtesy dr. ALJJ Bronckers),
which was found to be negative. 3H-proline was introduced in the
media by breaking ampoules at the beginning of the final 4 day culture period.
3.2.9 Histology
At day 4 of the final culture period the
samples used for histological evaluations were transferred from the incubator
to the glovebox. Fixative ampoules were broken and formaldehyde was released
into the culture bags at a final concentration of 1.26%. After fixation these
containers were placed in a 4°C cooler. At return in our laboratory in Amsterdam
the samples were dehydrated and embedded in Lowicryl K4M. 1 m sections
were made and stained with 0.2% toluidine blue without borax, pH=5.2, for one
minute at room temperature.
3.2.10 Groups and statistics
Samples were divided into 4 groups. Microgravity
(-g), in-flight 1g centrifuge, ground 1g, and ground
centrifuge 2g. Metatarsals were contralateral paired; -g
with in-flight 1g and ground 1g with ground 2g.
All measurements; lengths, glucose utilization,
relative collagen synthesis, mineral uptake (ED16), and mineral resorption (ED17.5)
were performed on the same metatarsal, or its culture medium.
Statistics were calculated using an analysis
of variance with the centrifuge position as a within subject factor and the
ground versus flight location as a between subject factor. All data are expressed
as mean ± SEM, n=8 for all groups unless indicated otherwise.
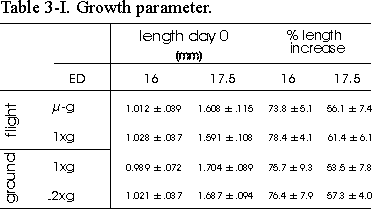
Table 3.I Growth parameter. Length of
ED16 and ED17.5 metatarsal long bones before and after the 4 day culture period.
Rudiments were contralateral paired: -g with in-flight 1g and
ground 1g with ground 2g. %Length increase is determined
as length (T4-T0)/T0100%. Data are
mean ± SEM, n=8, except for 16 day old microgravity, n=7.
3.2 RESULTS
Details of an ED16 and ED17.5 metatarsals
cultured for four days under microgravity conditions are shown in Fig. 3.3 and
3.4. The cells and surrounding matrix appeared quite healthy. No adverse effects
on cell integrity could be observed in the microgravity group as well as in
the other groups (data not shown).
All metatarsals showed a considerable increase
in length, as is shown in Table 3.I. There was an increase in %length of about
75% in ED16 and 55% in ED17.5 bones. No consistent differences were found between
the -g and in-flight 1g groups.
Glucose utilization is shown in Fig. 3.5.
The microgravity groups of both ED16 and ED17.5 metatarsals utilized significantly
less glucose compared to most other groups. The ED17.5 samples utilized more
than 3 times as much glucose compared to ED16 metatarsals.
Matrix formation, as measured by collagen
synthesis is shown in Fig. 3.6. Collagen synthesis was unchanged in the microgravity
groups of ED16 as well as ED17.5 metatarsals compared to any other group. There
is, however, a difference between both flight groups combined compared to both
ground groups combined in ED16 as well as ED17.5 metatarsals, p=0.064 and p=0.012,
respectively.
Mineralization was studied in the ED16 bones
by measuring the length of the calcified diaphysis, which at this stage of development
(ED16 plus 4 day culture) consists of a core of mineralized cartilage surrounded
by a primitive bone collar (Fig. 3.3). A 31.3% reduction in length of the diaphysis
in the microgravity group was found versus in-flight 1g (Figs. 3.1 and
3.7). There were no differences between microgravity and both ground groups.
The lengths of the diaphysis of both flight groups combined were significantly
larger compared to both ground groups combined (p=0.045).
Mineralization was also studied by measuring
the radioactive calcium and phosphate uptake in TCA extracted mineral of ED16
metatarsals (Fig. 3.8). A decrease of 46.4% in calcium uptake was found under
microgravity compared to the in-flight 1g. There was also a difference
in calcium uptake between the in-flight 1g and ground 1g groups.
Phosphorus followed the same pattern as calcium; a decreased uptake of 40.4%
under microgravity versus in-flight 1g.
Finally, mineral resorption was studied
in ED17.5 45Ca prelabeled bones (Fig. 3.9). In contrast to the decreased
mineral formation there was a pronounced increase of 42.7% in mineral resorption
under microgravity conditions versus in-flight 1g. No significant differences
were found between flight groups and either ground group.
Fig. 3.3 Photomicrograph of the
central part of an ED16 metatarsal cultured in microgravity for 4 days. CC=
calcified cartilage, HC= hypertrophic chondrocytes, PO= periosteum. Bar= 50
mm.
3.4 DISCUSSION
In this first experiment with fetal mouse
long bones cultured under microgravity conditions, we studied growth, glucose
utilization, collagen synthesis and mineral metabolism.
Four days of microgravity revealed no significant
differences in %length increase between -g and in-flight 1g
groups. There was, however, a slight tendency with ED17.5 metatarsals (p=0.064),
towards a reduced %length increase under microgravity compared to in-flight
1g as well as for ground 1g compared to 2g.
Duke et al. reported a significant reduction in length of the reserve
and hypertrophic/calcification zone in tibiae of rats flown for 12.5 days on
the Cosmos 1887,(17) although the more than 48 hours recovery time
before sacrificing the animals after this flight should be noted. Also, longitudinal
growth was impaired in the humerus(18) and proximal tibiae(4)
of rats flown for 7 days. The considerable increase in length in all groups
during the 4 day culture does emphasize favorable growth conditions in the culture
method used.
Fig. 3.4 Photomicrograph of the
central part of an ED17.5 metatarsal cultured in microgravity for 4 days. CC=
calcified cartilage, HC= hypertrophic chondrocytes, PO= periosteum, BC= primitive
mineralized bone collar. Note the excavation of the primitive marrow cavity
(MC). Bar= 50 mm.
We found a profound reduction of glucose
utilization under -g compared to in-flight 1g group in ED16
as well as ED17.5 metatarsals. An impaired glucose utilization was also found
in femoral-diaphyseal fragment explants from rats grown under simulated near
weightlessness.(19) On the other hand, an increased energy utilization
after cyclic loading was found by Skerry et al. and Dodds et al.
in vivo, and by El-Haj et al. in vitro. They reported an increase
in glucose-6-phosphate dehydrogenase activity in osteoblasts and osteocytes
after loading turkey and rat long bones.(20-22) The increased glucose
utilization by ED17.5 versus ED16 metatarsals is probably due to the larger
number of cells in the bigger ED17.5 rudiments.
In this study collagen synthesis was not
affected by microgravity. However, an increase in hydroxyproline concentration
in vertebrae has been found in rats after a 7 day spaceflight,(23)
and in rat intervertebral disks after a 14 day mission.(24) Duke
et al. reported wider collagen fibrils in the growth plate of rat tibia
flown aboard Cosmos 1887.(17) These changes in collagen fibers were
probably the result of changes in matrix proteoglycans, which were not studied
in the present investigation.
Our in vitro data on a reduced mineral
formation under microgravity are in agreement with earlier in vivo studies
under near weightlessness conditions.(1-5,7,8,25) The reduction in
length of the diaphysis, and the reduced calcium and phosphorus uptake indicate
an impairment of mineralization resulting from lack of the 1gravity
mechanical stimulation. The decreased total length of the ED16 microgravity
group at day 4 (0.067 mm, data not shown) can not account for the 0.124 mm reduction
in diaphyseal length (Fig. 3.7).
It has been suggested that mineralization
of metatarsals in vitro may be accelerated by shear stresses at the mineral/cartilage
interface.(26) Using a finite element analysis model it was, retrospectively,
concluded that these shear stresses are probably responsible for the increased
mineralization found in embryonic mouse long bones treated with intermittent
hydrostatic pressure.(9) It is obvious that in microgravity shear
stresses will not occur, since there are no mechanical forces acting. It will
be interesting to evaluate whether shear stresses play a role in the increased
mineralization of the in-flight 1g samples, compared to metatarsals
cultured under microgravity conditions.
Mineral uptake studies in skeletal tissues
are very complex because of the bone turnover. In the ED16 model, used for these
experiment, one starts with a non-mineralized metatarsal. Considering the fact
that the in vitro development always leaps behind in vivo growth,
the developmental stage for ED16 metatarsals plus 4 days culture is comparable
to an ED17 in vivo stage. This means that no osteoclasts or their precursors
have entered the mineralized diaphysis yet.(27,28) Histological observations
failed to show any osteoclasts in the mineralized zone of ED16 metatarsals after
4 days culture (Fig. 3.3). Also, the primitive marrow cavity as can be seen
in ED17.5 metatarsals (Fig. 3.4) is not observed in ED16 samples (Fig. 3.3).
Consequently, besides the always present physicochemical mineral exchange, there
is only de novo mineralization in these ED16 metatarsals, and no mineral
resorption.
Fig. 3.5 D-Glucose utilization
of ED16 and ED17.5 metatarsals, per rudiment during the final 4 days of culture.
Significance between groups is indicated by the same letter. a: p<0.05; b:
p<0.001; c,d,e: p<0.005.
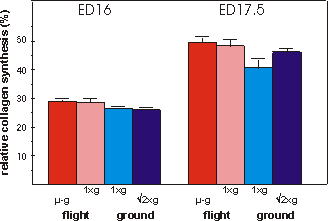
Fig. 3.6 Relative collagen synthesis (%) during the final 4 days in culture.
N=7 for ED17.5 in-flight 1g group.
In vivo
spaceflight studies are not equivocal with respect to bone resorption related
parameters. Some found no or a decrease (3,4), while others reported
an increase (5-8) in osteoclastic activity. Our in vitro results
show that osteoclastic mineral resorption is increased under microgravity versus
in-flight 1g. This is in agreement with extensive in vivo studies
by Whedon et al. and Leach et al., where an increased calcium,
phosphate and hydroxylysine excretion in astronauts during several Skylab missions
was reported, indicating an increased bone resorption.(29,30) The
clearly increased resorption found in the present study resulted from using
a more sensitive biological system and/or a more sensitive assay for osteoclastic
activity. And, since this was an in vitro organ culture, direct effects
were not obscured by systemic factors.
In the ED17.5 metatarsals, besides physicochemical
mineral exchange, probably only calcium release prevails in this model. What
is measured in this assay is only labeled calcium. The released 45Ca
in the medium, after culture, only derives from the in utero prelabeled
mineral. Cell mediated mineralization in this system hardly occurs since no
sodium--glycerophosphate was added to this medium. This is reflected
by the lack of increase in length of the mineralized diaphysis (Fig. 3.2). When
cell mediated re-uptake would take place, the calcium ions derive from the culture
medium pool which contains no 45Ca in the beginning, and only minute
amounts later in culture. The chance for a released 45Ca ion to be
incorporated again is insignificant since its concentration in the medium is
negligible compared to non-labeled calcium. Also, the amount of calcium incorporated
in the very actively mineralizing ED16 metatarsals is only ±1.1% of the total
medium calcium. Therefore, the maximum amount of released 45Ca to
be incorporated again probably would only have been a fraction of this 1.1%.
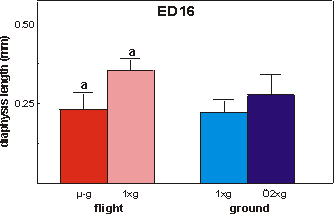
Fig. 3.7 Length of the calcified zone (diaphysis) of ED16 metatarsals after
the final 4 days of culture. Significance between groups is indicated by the
same letter. a: p<0.01.
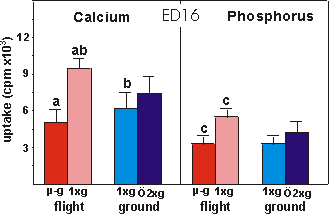
Fig. 3.8 Calcium and phosphorus uptake of the mineralized diaphysis of ED16
rudiments after the final 4 days of culture. Significance between groups is
indicated by the same letter. a,c: p<0.001; b: p<0.05.
In some parameters measured, differences
were found between the microgravity group and the in-flight 1g control,
whereas no such differences were found between the microgravity and the ground
1g groups. This poses the problem, which has emerged with the use of
on board 1g centrifuges, what to chose as the appropriate control group
in microgravity experiments. As discussed above, we have chosen for comparing
the microgravity and the in-flight 1g groups because these groups have
been subjected to the same launch related events and cosmic radiation. For that
purpose metatarsals of these groups were paired. For the ground 1g and
the ground centrifuge (2g) groups, which were not subjected
to the launch and flight related stimuli, we have used separate paired metatarsals.
The conclusion that in this in vitro model microgravity decreased glucose
utilization and matrix mineralization and increased mineral resorption is based
on comparing microgravity and in-flight 1g groups. However, there is
a possibility that exposure to microgravity of the in-flight 1g group
prior to culture on the on board 1g centrifuge (which is about 6 hours
in this experiment; see time-line) has sensitized the cells for the subsequent
change to 1g acceleration, while being stored at room temperature. This
means that the skeletal tissue cells indeed respond to microgravity, however
only in terms of becoming more sensitive to the following increase in acceleration.
Taken the ground 1g as control group it could be argued that microgravity
per se, as an ongoing event for 4 days, is ineffective to change mineralization
and resorption. It should be noted that this conclusion conflicts with many
in vivo findings which have shown a decreased mineralization under microgravity.(1-4)
If true, it could mean that the reported in vivo effects of microgravity
on skeletal tissues are indirect and the result of microgravity induced hormonal
changes.
Significant differences were found between
one or both flight groups and ground groups, in glucose utilization, relative
collagen synthesis, length of diaphysis and calcium uptake. These effects must
be due to launch characteristics, cosmic radiation and/or other local environmental
conditions, since these are the only differences. This implies that the metatarsals
are sensitive to these accelerations and vibrations during launch while stored
at room temperature and/or respond to the cosmic radiation while in orbit. This
emphasizes the importance of the use of an in-flight 1g centrifuge as
the best control to study microgravity effects.
In general, the in vitro experimental
approach to study the role of the mechanical environment in bone metabolism
is to apply loads upon cells and tissues. This is done for example by stretching
substrata,(31,32) or applying hydrostatic pressure,(9,11,22)
fluid shear stresses(33) and hypergravity.(10,34) The
question can be raised whether the control, non-stressed, conditions on Earth
are indeed totally unloaded (disuse), or whether the on Earth always present
1gravity acceleration is a situation of stress per se. The only
possibility to decrease mechanical load in vitro is to reduce the unit
gravity vector which acts upon cultures. Microgravity provides this unique condition,
in which cells and tissues can be studied in the lowest possible mechanical
stress environment. Since the differences found in this experiment are due to
a decrease of the gravitational force by the reduction of 1g to microgravity,
it is obvious that a 1g condition is a situation of loading. Therefore
on Earth, all objects, and thus cells, are inherently subjected to this load.
Some cells and tissues are even responding to this acceleration as we have shown
in this paper.
Summarizing we conclude that fetal mouse
metatarsal long bones in vitro are sensitive to the near absence of gravity.
Metatarsals respond, during a 4 day culture period under microgravity, with
a decreased glucose utilization, a decreased calcification and an increased
mineral resorption, while overall growth and collagen synthesis are not affected.
These are effects of microgravity on skeletal tissues only, and are not mediated
by changes in systemic factors. The fetal mouse metatarsal long bone is a sensitive
model for further studies of the cellular mechanisms involved in the changed
metabolism of skeletal tissues under microgravity.
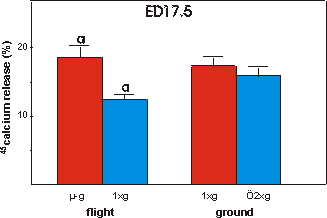
Fig. 3.9 Percentage release of 45calcium from ED17.5 metatarsals
after the final 4 days of culture. Significance between groups is indicated
by the same letter. a: p<0.01.
3.5 REFERENCES
1 Vose GP 1974 Review of roentgenographic
bone demineralization studies of the gemini space flights. Am. J. Roentgenol.,
Rad. Therapy & Nuclear Med. 121:1-4.
2 Oganov VS, Rakhmanov AS, Novikov
VE, Zatsepin ST, Rodionova SS, Cann Ch 1991 The state of human bone tissue during
space flight. Acta Astronautica 23:129-133.
3 Morey ER, Baylink DJ 1978 Inhibition
of bone formation during space flight. Science 210:1138-1141.
4 Vico L, Novikov VE, Very JM, Alexandre
C 1991 Bone histomorphometric comparison of rat tibial metaphysis after 7 day
tail suspension vs. 7 day spaceflight. Aviat. Space Environ. Med. 62:26-31.
5 Kaplansky AS, Durnova GN, Burkovskaya
TE, Vorotnikova EV 1991 The effect of microgravity on bone fracture healing
in rats flown on Cosmos 2044. Physiologist 34:S196-S199.
6 Vico L, Chappard D, Alexandre C,
Palle S, Minaire P, Riffat G, Novikov VE, Bakulin AV 1987 Effects of weightlessness
on bone mass and osteoclast number in pregnant rats after a five-day spaceflight
(Cosmos 1514). Bone 8:95-103.
7 Földes I, Rapcsák M,
Szilágyi T, Organov VS 1990 Effects of space flight on bone formation
and resorption. Acta Physiol. Hung. 75:271-85.
8 Zerath E, Holy X, Malouvier A,
Caissard JC, Nogues C 1991 Rat and monkey bone study in the biocosmos 2044 space
experiment. Physiologist 34:S194-S195.
9 Klein Nulend J, Veldhuijzen JP,
Burger EH 1986 Increased calcification of growth plate cartilage as a result
of compressive force in vitro. Arthritis Rheum 29:1-9.
10 Van Loon JJWA, Veldhuijzen JP,
Burger EH 1990 Hypergravity and bone mineralization. Proc. Fourth Europ. Symp.
on Life Sci. Res. in Space, ESA SP-307:393-396.
11 Klein Nulend J, Veldhuijzen JP,
Strien ME van, Jong M de, Burger EH 1990 Inhibition of osteoclast bone resorption
by mechanical stimulation in vitro. Arthritis Rheum 33:66-72.
12 Mesland D, Accensi A, Alfermann
C, Bennett J, Chin D, Foeng A, Franz A, Gesta-Fernandez J, Goldzahl N, Helmke
H, Ives J, Kruit A, Soons A, Burden D, Millican S 1987 The Biorack facility
and its performance during the D1 spacelab mission. ESA publication SP-1091,
9-26.
13 Van Loon JJWA, Veldhuijzen JP,
Windgassen EJ, Brouwer T, Wattel K, Vilsteren van M, Maas P 1994 Development
of tissue culture techniques and hardware to study mineralization under microgravity
conditions. Adv. Space Res.: 14:289-298.
14 Peterkofsky B, Diegelmann R 1971
Use of mixture of proteinase- free collagenase for the specific assay of radioactive
collagen in the presence of other proteins. Biochemistry 10:988-994.
15 Kream BE, Smith MD, Canalis E,
Raisz LG 1985 Characterization of the effect of insulin on collagen synthesis
in fetal rat bone. Endocrinology 116:296-302.
16 Diegelmann RF, Peterkofsky B 1972
Collagen synthesis during connective tissue
development in chick embryo. Develop.
Biol. 28:443-453.
17 Duke PJ, Durnova G, Montufar-Solis
D 1990 Histomorphometric and electron microscopic analyses of tibial epiphyseal
plates from Cosmos 1887 rats. FASEB J. 4:41-46.
18 20 Shaw SR, Vailas AC, Grindeland
RE, Zernicke RF 1988 Effects of a 1-wk spaceflight on morphological and mechanical
properties of growing bone. Am. J. Physiol 254:R78-R83.
19 Yamagushi M, Ozaki K, Hoshi T
1991 Simulated weightlessness and bone metabolism: impairment of glucose consumption
in bone tissue. Res Exp Med 191:105-111.
20 Skerry TM, Bitensky L, Chanyen
J, Lanyon LE 1989 Early strain-related changes in enzyme activity in osteocytes
following bone loading in vivo. J Bone Min Res 4:783-788.
21 Dodds RA, Ali N, Pead MJ, Lanyon
LE 1993 Early loading-related changes in the activity of glucose-6-phosphate
dehydrogenase and alkaline phosphatase in osteocytes and periosteal osteoblasts
in rat fibulae in vivo. J Bone Min Res 8:261-267.
22 El Haj AJ, Minter SL, Rawlingson
SCF, Suswillo R, Lanyon LE 1990 Cellular responses to mechanical loading in
vitro. J Bone Min Res 5:923-932.
23 Patterson-Buckendahl P, Arnaud
SB, Mechanic GL, Martin RB, Grindeland RE, Cann CE 1987 Fragility and composition
of growing rat bone after one week in spaceflight. Am. J. Physiol. 252:R240-R246.
24 Pedrini-Mille A, Maynard JA, Durnova
GN, Kaplansky AS, Pedrini VA, Chung CB, Fedler-Troester J 1992 Effects of microgravity
on the composition of the invertebral disk. J. Appl. Physiol. 73(2):26S-32S.
25 Simmons DJ, Grynpas MD, Rosenberg
GD 1990 Maturation of bone and dentin matrices in rats flown on the Soviet biosatellite
Cosmos 1887. FASEB J. 4:29-33.
26 Wong M, Carter DR 1990 Theoretical
stress analysis of organ culture osteogenesis. Bone 11:127-31.
27 Burger EH, Van Der Meer JWM, Van
De Gevel JS, Gribnau JC, Thesingh CW, Van Furth R 1982 In vitro formation of
osteoclasts from long-term cultures of bone marrow mononuckear phagocytes. J.
Exp. Med. 156: 1604-1614.
28 Scheven BAA, Kawilarang-de Haas
EWM, Wassenaar A-M, Nijweide PJ 1986 Differentiation kinetics of osteoclasts
in the periosteum of embryonic bones in vivo and in vitro. The anatomical record
214: 418-423.
29 Whedon GD, Lutwak L, Reid J, Rambaut
P, Whitte M, Smith M, Leach C 1974 Mineral and nitrogen metabolic studies on
Skylab orbital space flights. Trans Assoc. Am. Physicians 87:95-110.
30 Leach CS, Rambaut PC 1977 Biochemical
responses of the Skylab crewmen: an overview. In: Johnston and Dielein (ed)
Biomedical results from Skylab. NASA, Washington DC, USA, SP-377, 204-216.
31 Somjen D, Binderman I, Berger
E, Harell A 1980 Bone remodelling induced by physical stress is prostaglandin
E2 mediated. Biochimica et Biophysica Acta 627:91-100.
32 McDonald F, Houston WJB 1992 The
effect of mechanical deformation on the distribution of potassium ions across
the cell membrane of sutural cells. Calcif Tissue Int 50:547- 552.
33 Reich KM, Frangos JA 1991 Effect
of flow on prostaglandin E2 and inositol triphosphate levels in osteoblasts.
Am. J. Physiol. 261:C428-C432.
34 Inoue H, Hiasa K, Samma Y, Nakamura
O, Sakuda M, Iwamoto M, Suzuki F, Kato Y 1990 Stimulation of proteoglycan and
DNA syntheses in chondrocytes by centrifugation. J. Dent. Res. 69:1560-1563.
3.6 ACKNOWLEDGMENTS
We like to thank the whole Biorack-team
of ESA, STS-42 crew, Bionetics and NASA-KSC for their excellent support. Statistical
support by Mr. FC van Ginkel and HJ Ader is greatly acknowledged. This work
was supported by the Space Research Organization of the Netherlands (SRON) grant
MG-004, and by the Dutch Organization of Scientific Research (NWO) grant 900-541-133.




