Polysulphone Inhibits Final Differentiation Steps of Osteogenesis
In Vitro.
Jack J.W.A. van Loon1, Johan Bierkens2,
Jef Maes2, Greet E.R. Schoeters2, Daniella Ooms2,
Behrouz Zandieh Doulabi1, J.Paul Veldhuijzen1.
1 Academic Centre for Dentistry Amsterdam (ACTA),
Dept. of Oral Cell Biology, Amsterdam, The Netherlands.
2 Flemish Institute for Technological Research (VITO), Environmental
Division, Mol, Belgium.
ABSTRACT
Biocompatibility is an important factor in the development
of orthopaedic implants as well as in the development of new tissue culture
devices.
Polysulphone has been used for orthopaedic implants because
of its mechanical properties, ease of sterilization, its molding capacity and
its biocompatibility. Therefore polysulphone has been chosen as the prime material
for the construction of tissue culture devices to be used for cultivation of
osteogenic cells (pre-osteoblast-like MN7 cells and primary bone marrow fragments)
as well as complete fetal long bone explants under space flight conditions.
Whereas polysulphone did not interfere with the proliferation
in early stages of bone forming cells, we show that leacheble factors within
the polysulphone polymer prevented the final steps of matrix formation as measured
by collagen synthesis and matrix mineralization. These data argue against polysulphone
is a material for orthopaedic implants.
Key words: polysulphone, bone, mineralization, orthopaedic
implants, microgravity, tissue culture device.
This paper is submitted for publication.
4.1 INTRODUCTION
Recently carbon fiber reinforced polysulphone has attracted
some attention as a new implant material in tumor surgery of the spine,(1)
internal fracture fixation of long bones(2) or after auto-alloplastic
tooth replantation.(3) Apart from its improved mechanical properties
as compared with metallic alloys, also the effects on a biological system seems
to be favorable.(4) Polysulphone is also promising from an engineering
point of view, since it is translucent, very well machinable and can be sterilized
by heat, gas, or X-rays.(5) For these reasons polysulphone (PSU)
was chosen as the prime material for constructing tissue culture devices. These
devices are to be used in experiments to study the effect of microgravity on
bone development and metabolism while in culture.
It has been shown that bone metabolism changes during space
flight.(6,7) It is not clear from these in vivo experiments,
however, whether these changes are due to changes in systemic factors like hormone
levels or body fluid shifts,(8) or whether they are the result of
direct effects of near weightlessness on bone cell metabolism. By using these
automated devices for in vitro studies we might address this problem
on a more basic level.
Intramembranous ossification is characterized by a number
of successive steps. Initially quiescent bone precursor cells are recruited
for proliferation (proliferation phase), whereafter they gradually acquire bone
phenotypic markers (maturation phase). In order of their expression these main
markers are: Collagen Type I, alkaline phosphatase, osteopontin, and osteocalcin.(9)
The final result of this differentiation process is the formation of nucleation
sites and finally a fully mineralized bone matrix (calcification phase). In
the present experiments studying the effect of PSU on osteogenic differentiation,
cell number, collagen synthesis and calcification were chosen as markers for
the proliferation, maturation and calcification phase, respectively.
To gain better insight in the effect of PSU on the different
osteogenic differentiation phases, three distinct but complementary bone differentiation
models have been tested in contact with PSU. In the first model osteoblast precursor
cells in primary murine bone marrow fragments have been triggered to form a
mineralized matrix de novo10) The second model features a
c-fos transformed cell line with pre-osteoblast like characteristics (MN7),
in order to study its development towards a more mature osteoblastic phenotype.(11)
In the last model, complete explants, 16 day old fetal mouse cartilaginous long
bone rudiments (metatarsals), have been used to study endochondral and intramembranous
calcification in vitro. Whereas in the former two models the recruitment
and maturation of pre-osteoblasts towards mature osteoblasts could be studied,
the latter model focusses on the final differentiation step in the formation
of bone, i.e. cartilage and bone calcification.
Besides the variation in developmental stages, these osteogenic
models have also been chosen to be used for future microgravity experiments,
since it has already been demonstrated that osteoblast like cells (12)
as well as metatarsal long bones(13) are susceptible to changes in
gravitational forces.
The results have demonstrated that parameters indicative for
cell proliferation have not been affected by PSU. However, the maturation and
mineralization steps have been shown to be significantly retarded in the presence
of PSU in all tests performed. We discuss the possible implications of these
results on the potential use of PSU as an implant material.
4.2 MATERIALS AND METHODS
4.2.2 Tissues
4.2.2.1 Bone marrow fragments
The establishment of bone marrow organ cultures has been described
previously.(10) Briefly, the femurs of three-month-old Balb/c inbred
mice (VITO animal facility, Belgium) were dissected, cleaned and the ends separated
from the midshaft. The marrow was carefully flushed from the midshaft with a
22-gauge needle connected to a syringe. The marrow was aseptically collected
as marrow fragments without disrupting its original three dimensional structure.
The marrow fragments were allowed to adhere to aseptic collagen supports (CollaTech
Inc.) for 30 minutes, whereafter 1 ml of BGJb medium (Gibco) was added, supplemented
with 10% fetal calf serum, 1% L-glutamine and 1% gentamicin (all derived from
Gibco), 10 mM sodium--glycerophosphate and 50 g/ml L-ascorbic
acid (Sigma). The cultures were incubated in a 5% CO2 in air, 37°C,
100% relative humidity incubator. The tissue culture medium was changed every
3 days. Control experiments were performed in standard laboratory 24 wells plates.
The mineralization rate of bone marrow cultures was followed
in time using 85Sr (5 Ci/ml)(NEN) as a tracer for calcium.(10)
85Sr gamma-radioactivity in thoroughly rinsed marrow fragments, was
measured using a NaI(Tl) detector (Packard 5320).
4.2.2.2 MN7 monolayer cultures
Establishment of the MN7 preosteoblast-like cell line has
been published elsewhere.(11) Adherent cell cultures were established
by plating 103 cells/well in 96 well plates (Gibco). Cell number
was scored on day 3 with a particle counter (Coulter model ZF, Coulter Electronics).
Medium and culture conditions were identical to those described for the bone
marrow fragments.
4.2.2.3 Metatarsal long bone rudiments
The middle 3 cartilaginous metatarsal long bones in each foot
of 16 day embryonic (ED16) Swiss mice were used. Rudiments (typical dimensions
1.00.3 mm) were aseptically harvested and cultured in bicarbonate buffered
MEM without nucleosides supplemented with 50 mg/l gentamicin and 0.5%
v/v fungizone (Gibco), 50 mg/l ascorbic acid, 300 mg/l glutamine (Merck), 0.2%
w/v BSA Factor V and 1 mM Na--glycerophosphate (Sigma). Controls were
always cultured in standard 24 wells tissue culture plates (300 l medium
/ well) (Greiner) and placed in a 5% CO2 in air, 37C, 100%
relative humidity incubator. ED16 metatarsal long bones are cartilaginous non-mineralized
rudiments at dissection but will calcify while in culture. Using an inverted
microscope, growth and mineralization were assessed as total length of the rudiment
and the calcified zone (diaphysis), respectively. Metatarsals were contralateral
paired, one bone receiving control and the other experimental treatment.
4.2.3 Hardware
4.2.3.1 Tissue Culture Device
Prototype hand operated devices based on a design by Ubbels and CCM*
were constructed of PSU (Ensinger, Technische Kunststoffe, Nufringen, Germany).(14)
Each device held two culture compartments. The devices also contained reservoirs
for fluid exchange in the culture compartment (3 per culture compartment), each
of them containing 1.0 ml tissue culture medium or fixative. This allowed for
two successive replenishment of the tissue culture medium and a final fixation
of the biological samples. Each of the two culture compartments contained either
one bone marrow fragment, or 4 metatarsal long bones in 1.0 ml of culture medium.
The surface area contact of PSU with culture medium in the culture compartment
was 550 mm2. The culture compartment was covered with a gas permeable
polyethylene foil to allow ample gas exchange (5% CO2 in air) during
cultivation. (Fig. 4.1)
*CCM, Center for Construction and Mechanotronics,
Nuenen, The Netherlands.
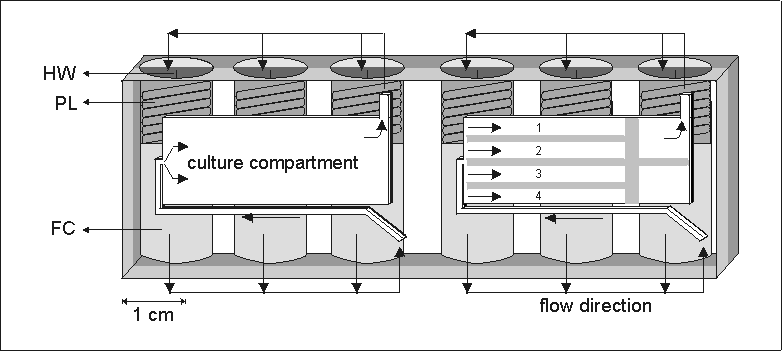
Figure 4.1: A schematic representation of an automated tissue culture device
(204080 mm (lwh)) made out of a single block
of polysulphone. The actual culture compartment is 28133 mm.
It has been used for bone marrow cultures (left compartment) or for four metatarsal
long bones (right compartment with separators). For each culture compartment
fresh culture media or fixatives were stored in three fluid reservoirs (FC).
The fluid was forced to the culture compartment by the release of a spring loaded
plunger (PL) activated by scorching a nylon string via a dedicated heat wire
(HW). The fluid was lead to the cultures via a system of internal channels and
valves, indicated by arrows. The spend medium was forced out of the culture
compartment and found its way to the void space behind the released plunger.
The culture compartment was covered by a gas permeable polyethylene membrane.
4.2.4 Techniques
4.2.4.1 Collagen assay
Collagen synthesis was measured by the incorporation of 3H-proline
(Amersham, UK) into collagenase digestible protein (CDP).(15) The
collagenase (Worthington, New Jersey) was free of non-specific protease activity.
The cell cultures were exposed to 3H-proline (1 Ci/ml) for
the last 18 hours of culture and then washed three times with PBS in 35 mm petri
dishes. Collagenase was added (0.1 g/ml in PBS) for 3 hours and the
3H was measured in a liquid scintillation counter.
4.2.4.2 Thymidine assay
Proliferation of bone marrow and MN7 cells was measured by
the incorporation of tritiated thymidine. The thymidine was added to the culture
medium 16 hrs before finishing the experiment. After culture, the cells were
washed and treated with trypsin, harvested and collected onto a paper support
(Glass fiber filter, Beckman). Subsequently, the filters were washed, punched
out and the remaining radioactivity was counted in a liquid scintillation analyzer
(Tri-Carb 1600 CA, Packard).
4.2.4.3 Cytotoxicity assays
To study the cytotoxicity of PSU two methods were used:
1: Extraction method one:
This method for studying the cytotoxicity of plastics has
been described earlier.(16) Briefly, for this conditioned medium
(CM-1) the PSU resin was fragmented with a lathe to curled shavings. Eight grams
of these shaving were autoclaved in 40 ml of Milli-Q water. The extraction water
was 1:1 mixed with 2 times concentrated complete culture medium. This CM-1 was
used for culturing bone marrow fragments as well as long bone rudiments.
2: Extraction method two:
To study whether leacheble factors deriving from PSU affects
metatarsal long bone development, a second conditioned medium, CM-2, was prepared
by incubating plates of PSU (total surface area 330 mm2) in a standard
6 wells tissue culture plate (polystyrene, Greiner) in 2 ml complete culture
medium (MEM + all additives) for 3 days at 37C in a 5% CO2
incubator. Control media were without PSU plates. This CM-2 was subsequently
used to culture ED16 metatarsals for 6 days.
For CM-1 and CM-2 cultures, both proliferation and differentiation
characteristics were measured.
4.2.5 Histology
Some cell and tissue samples were fixed with formaldehyde
and embedded in historesin for histological evaluation. 3 m sections
were stained with either toluidine blue (0.2% + 0.2% borax, 90 sec.) for general
histology and cell integrity, or alizarin red-S (1%, pH=5.5, 60 sec., room temp.)
for mineral formation.
4.2.6 Anion / Cation composition
Changes in anion and cation compositions were measured in
culture medium. Complete BGJb tissue culture medium, including all additives,
was incubated in open PSU trays or control petri dishes for 3 days in a 37C,
5% CO2 incubator. The ionic composition of the medium was determined
with a plasma emission spectrometer (Jarrell-ASM, Atomcomp, model 750).
4.2.7 Statistics
Statistics were calculated using the two tailed Student t-test,
for paired or unpaired values when applicable. All data are expressed as means
± SEM.
4.3 RESULTS
Figure 4.1 shows the modified version of a completely automated
tissue culture device, which was already successfully used on board the Space
Shuttle and MASER sounding rocket missions.(14,17) The modified device
has been developed for studying osteogenic cells in near weightlessness. Hand-operated
prototypes of these cell culture devices have been used for biocompatibility
tests. In these prototypes the proliferation of cells in bone marrow fragments
and 16 day old metatarsal long bones as measured by 3H-thymidine
incorporation and total bone length, respectively, was suppressed as compared
to standard laboratory tissue culture plastics (Figs. 4.2a and 4.2b). In the
same experiments the final differentiation steps, i.e. collagen synthesis
and calcification were greatly reduced or totally absent (Figs. 4.2c and 4.2d).
To rule out 'device effects' (i.e. geometrical and
physicochemical interferences from the integrated device on the osteogenic cultures),
experiments were performed in open culture trays made from PSU. The use of these
open PSU trays resulted in large variation among the samples (Fig. 4.3). There
is, however, a significant reduction of strontium uptake in bone marrow fragments
cultured in PSU compared to controls. Similar results were obtained when the
PSU CM-1 was used to cultivate osteogenic cells and tissues. A significant decrease
has been observed in the acquisition of collagen and in mineralization, in the
bone marrow fragments and metatarsal long bones, respectively, when cultured
in CM-1 medium (Fig. 4.5c, 4.5d). In all three different bone cell differentiation
models that we used, no significant effect of PSU has been seen on the initial
proliferative stages of the cultures (Figs. 4.4, 4.5a and 5.5b).
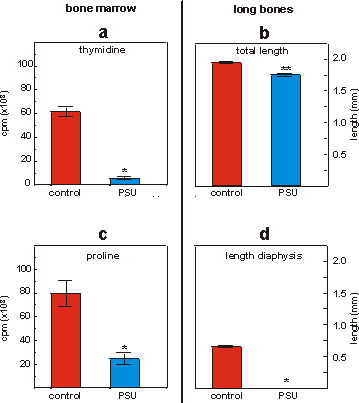
Figure 4.2: 3H-thymidine (a) and 3H-proline (c) incorporation
in mineralizing bone marrow fragments cultured on collagen matrices in standard
multiwell plates or PSU tissue culture devices. Values are means ± SEM, n=4,
*p<0.001. Total length (b) and length of the calcified zone (diaphysis, d)
of fetal mouse metatarsal long bones cultured for 6 days in standard multiwell
plates or PSU tissue culture devices. Values are means ± SEM, n=8, *p<0.001,
**p<0.0001.
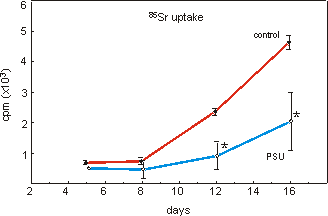
Figure 4.3: 85Sr uptake in mineralizing bone marrow fragments cultured
on collagen matrices in open PSU trays. Data are means ± SEM, n=4, *p<0.05.
Also, from histological sections (Figs. 4.6a and 4.6b), it
is clear that when metatarsal long bone rudiments are cultured for 6 days in
either control or PSU conditioned medium no cell death or necrosis can be detected.
When stained for calcium by alizarin red-S, however, mineralization of the matrix
is only present in control cultures (Fig. 4.6c).
After incubation of complete BGJb tissue culture medium in
either multiwell plates or open PSU trays, no striking changes were found of
either cation or anion concentrations as a result of adsorption or excretion
of these two polymers measured by plasma emission spectrometry (Table 4.I).
The control medium, incubated in multiwell plates, even showed a decreased calcium
and phosphate content compared to untreated or PSU incubated medium. The PSU
extract showed a significant increase in Ca and P ion content compared to fresh
medium. Measurements have been partly biased by the large amounts of sodium.
Also no increase in Cd, Co, Cu, Fe, Pb, Se, Zn or Mn contents were detected
after incubation.
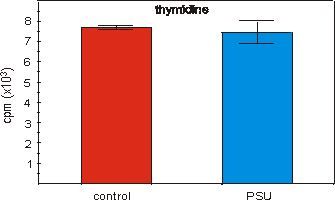
Figure 4.4: 3H-thymidine incorporation in MN7 cells cultured for
3 days in medium derived from PSU extracted water (CM-1). Values are means ±
SEM, n=10.
4.4 DISCUSSION
With the ageing population in the Western world and the expansion
of human activities towards space, questions related to bone metabolism and
its disorders have come to the foreground. Whereas brittleness of the bone caused
by immobility, old age or metabolic disease requires the development of more
and better bone implant materials, microgravity induced osteoporosis has to
be understood before actual long term manned space flight can be planned. Both
issues, at first sight unrelated, merge because they both rely on thorough biocompatibility
testing. In both bone implant surgery as well as in the development of a tissue
culture device, only those materials can be used which are biocompatible and
which allow maximal differentiation of skeletal tissues and cells. The present
experiments have been conducted in order to develop an automated cell culture
unit to study microgravity induced changes in bone metabolism. A lesson can
be learned as far as the use of test systems required for biocompatibility testing
for implant materials in vitro is concerned.
Although, it is well known that different cell types and various
stages of cell development react differently to drugs or bioeffector molecules,
some biocompatibility experiments still rely on relatively undifferentiated
cells such as fibroblasts.(18,19,20) These in vitro tests
do not always reflect the cell population at the in vivo application
site of the biomaterials tested. For this in vitro study we used representatives
of various stages in bone development therefore approaching the in vivo
situation for orthopaedic implants as close as possible.
In the osteogenic models used, we have found no PSU induced
retardation in proliferation of MN7 cells (Fig. 4.4), bone marrow fragments
(Fig. 4.5a) or general growth (= total length) of ED16 metatarsal long bones
(Fig. 4.5b). These results have been confirmed via histological examinations
of bone marrow fragments (data not shown) and whole explants (Fig. 4.6a, 4.6b).
In either case only minimal or no cell death has been observed (Figs. 4.6a and
4.6b). These observations are consistent with previous experiments studying
the proliferation of fibroblasts in PSU conditioned medium.(4) The
same authors also showed that cell death due to PSU, as accessed by lactate
dehydrogenase (LDH) concentration in the medium, did not occur.
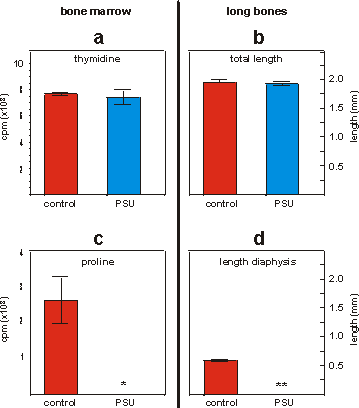
Figure 4.5: 3H-thymidine (a) and 3H-proline (c) incorporation
in mineralizing bone marrow fragments cultured on collagen matrices in conditioned
medium derived from normal multiwells (control) or PSU resin after 9 days in
culture (CM-2). Data are means ± SEM, n=4, *p<0.01. Total length (b) and
length of the calcified zone (diaphysis, d) of fetal mouse metatarsal long bones
cultured in multiwell or PSU conditioned medium (CM-2 respectively, for 6 days.
Data are means ± SEM, n=8, **p<0.0001.
In contrast with cell proliferation during the initial stages
of cultivation, the acquisition of more mature phenotypic characteristics of
osteogenesis (collagen synthesis and mineralization) were clearly shown to be
inhibited by PSU (Figs. 4.2c, 4.2d, 4.3, 4.5c and 4.5d). Indirect effects, related
to PSU, such as inhibition of collagen synthesis and calcification by depletion
of the tissue culture medium of proteins and/or minerals required for mineralization
have to be ruled out. Firstly Mandenius et al.(21) showed
that only minute amounts of proteins adsorb onto PSU surfaces under physiological
conditions. Secondly our analysis of cat- and anions composition of tissue culture
medium has shown no dramatic PSU induced changes during a three day incubation
(Table 4.I). Moreover, since not only PSU devices, but also PSU extracts (CM-1
and CM-2) interfere with the osteogenic differentiation, leacheble components
are the only likeable candidates for interference (Figs. 4.5c and 4.5d). Since
these components have been shown not to effect cell proliferation they have
to be considered non-cytotoxic. The inhibition of osteogenesis partly derives
from decreased collagen synthesis and/or post excretion modulation of molecules
or molecule complexes in the extracellular matrix. The mechanism by which PSU
interferes with the differentiation of skeletal cells and their extracellular
matrix has to be further investigated.
Figure 4.6: Histology (a) of fetal mouse metatarsal long bones (ED16) cultured
for 6 days in conditioned medium (CM-2) from standard multiwell plates (polystyrene)
or PSU resin, respectively. a: Control bone, stained with toluidine blue (TB).
b: Cultured in PSU conditioned medium (CM-2), stained for TB. c: Central diaphysis
of the metatarsal long bone cultured in multiwell conditioned medium (CM-2),
stained for alizarin red-S. d: Highly overexposed photo of the central diaphysis
of a metatarsal long bone cultured in PSU CM-2, stained for alizarin red-S.
Bar = 100 mm.

Table 4.I: Plasma emission spectrometer analysis of BGJb culture medium including
additives (fresh medium) after three days incubation in a 37C incubator
in either normal multiwells (Multiwell) or open PSU plates (PSU). Concentrations
are expressed as ppm and are presented as means ± SEM. Significance between
groups is indicated by the same letter. a,b: p<0.01, c,d: p<0.0001, e,f:
p<0.05.
The experiments being discussed were performed with the aim
to develop a biocompatible tissue culture device to be used for space flight
experiments on bone development and metabolism, and were entirely performed
in vitro. These data can contribute, however, to our understanding of
in vivo effects seen with PSU containing implants. Although PSU was successfully
used as bone implant material,(22) a thin layer of non-mineralized
fibrous tissue at the bone-PSU implant interface has been reported by Knowles
et al.,(23) which was not seen in hydroxyapatite / polyhydroxybutyrate
implants. Leacheble factors from PSU could explain this phenomenon. These leacheble
factors may have a profound impact on the final choice of PSU as implant material.
Although, in vitro methods with fibroblasts may be suitable first indicators
for a general biocompatibility testing of new materials, future studies should
aim at mimicking the in vivo situation more closely. More differentiated
skeletal cells and tissues and relevant parameters should be introduced, such
as described by Puleo et.al or Angle et al.,(24,25)
when orthopaedic implants are considered.
From these biocompatibility tests it is also clear that the
application of similar hardware already successfully used for previous space
flight experiments with Xenopus amphibian eggs(14) and human A431
epidermoid carcinoma cells(17) does not necessarily imply favorable
culture conditions for other biological systems. This PSU material can not be
used as prime material for devices to study the various differentiation steps
in skeletal tissues under space flight conditions.
With the use of bone marrow fragments, osteogenic cell lines
or developing fetal mouse metatarsal long bones, the in vivo aspects
of skeletal growth, maturation and calcification is simulated more closely.
These three osteogenic models used in this study may be considered to be used
for future biocompatibility tests for orthopaedic implants.
4.5 CONCLUSION
In order to allow autonomous cultivation of mammalian cells
in space, a fully automated tissue culture device has been developed which has
allowed successive refreshment of the spent culture medium and final fixation
of cells. We have shown that when these devices are made of polysulphone, leacheble
components inhibit the final steps of osteogenesis. In the context of the interest
that exists for PSU as an implant material, the present data may urge for some
caution.
4.6 REFERENCES
1. C. Burri, L. Claes and O. Wörsdörfer "Osteosynthese
an der Wirbelsäule mit individuell gearbeiteter Platte aus kohlenstoffaserverstärktem
Polysulfon," Unfallchirurg, 89, 528-532 (1989).
2. L. Claes, "Kohlenstoffaserverstärktes Polysulfon: ein
neuer Implantatwerkstoff," Biomed. Technik, 34(12), 315-319 (1989).
3. W. Foerster, W. Huttner and H. Kirschner, "Kohlenstoffaserverstärktes
Polysulfon als Implantatmaterial; Werkstoffliche Eigenschaften und biologische
Untersuchung," Dtsch Z Mund Kiefer GesichtsChir, 8, 437-440 (1984).
4. L.M. Wenz, K. Merritt, S.A. Brown, A. Moet and A.D. Steffee,
"In vitro biocompatibility of polyetheretherketone and polysulfone composites,"
J. Biomed. Mater. Res., 24, 207-215 (1990).
5. A.K. van der Vegt, in Polymeres: from chain to resin,
Delfsche Uitgevers Maatschappij, Delft, The Netherlands (in Dutch) 1991.
6. G.P. Vose, "Review of roentgenographic bone demineralization
studies of the gemini space flights," Am. J. Roentgenol., Rad. Therapy &
Nuclear Med., 121, 1-4 (1974).
7. E.R. Morey and D.J. Baylink, "Inhibition of bone formation
during space flight," Science, 210, 1138-1141 (1978).
8. C.S. Leach and P.C. Rambaut, "Biochemical responses of skylab
crewmen: an overview," in Biomed results from skylab, R.S. Johnston,
L.F. Dietlein (ed.), NASA SP-377, NASA, Washington, D.C. U.S.A., 1977, pp. 204-216..
9. G.S. Stein, J.B. Lian and T.A. Owen, "Relationship of cell
growth to the regulation of tissue-specific gene experession during osteoblast
differentiation," FASEB J., 4, 3111-3123 (1990).
10. G.E.R. Schoeters, L. de Saint-Georges, R. Van Den Heuvel
and O. Vanderborght, "Mineralization of adult mouse bone marrow in vitro," Cell
Tissue Kinetics, 21, 363-374 (1988).
11. E. Mathieu, G. Schoeters, F. Vander Plaetse and J. Merregaert,
"Establishment of an Osteogenic cell line derived from adult mouse bone marrow
stroma by use of recombinant retrovirus," Calcif. Tissue Int., 50, 362-371
(1992).
12. M. Miwa, O. Kozawa, H. Tokuda, A. Kawakubo, M. Yoneda,
Y. Oiso and K. Takatsuki. "Effects of hypergravity on proliferation and differentiation
of osteoblast like cells," Bone and Mineral, 14, 15-25 (1991).
13. J.J.W.A. van Loon, J.P. Veldhuijzen and E.H. Burger, "Hypergravity
and bone mineralization," in Proc. Fourth Europ. Symp. on Life Sci. Res.
in Space, ESA SP-307, V. Davis (ed.), ESA Publication Division, ESTEC,
Noordwijk, The Netherlands, 1990, pp. 393-396.
14. G.A. Ubbels, "The role of gravity in the establishment
of the dorso/ventral axis in the amphibian embryo," in: Biorack on Spacelab
D1, N. Longdon, V. David (ed.), ESA Publication Division, ESTEC, Noordwijk,
The Netherlands, ESA SP-1091, 1988, pp. 147-155.
15. B. Peterkofsky and R. Diegelmann, "Use of mixture of proteinase-
free collagenase for the specific assay of radioactive collagen in the presence
of other proteins," Biochemistry, 10, 988-994 (1971).
16. D.E. Andersen and V. Nielsen, "In vitro screening for acute
cytotoxicity on plastic materials," 5th Int. workshop on in vitro toxicology.
Schloss Elmau, F.R.G., 2-4 (1988).
17. R.P. de Groot, P.J. Rijken, J. den Hertog, J. Boonstra,
A.J. Verkleij, S.W. de Laat and W. Kruijer, "Microgravity decreases c-fos induction
and serum response element activity," J. Cell Sci., 97, 33-38 (1990).
18. K. Iio, N. Minoura, S. Aiba, M. Nagura and M. Kodama, "Cell
growth on poly(vinyl alcohol) hydrogel membranes containing biguanido groups,"
J. Biomed. Mater. Res., 28, 459-462 (1994).
19. J.C. Wataha, C.T. Hanks and R.G. Craig, "In vitro effects
of metal ions on cellular metabolism and the correlelation between these effects
and the uptake of the ions," J. Biomed. Mater. Res., 28, 427-433 (1994).
20. A. van Sliedregt, J.A. van Loon, J. van der Brink, K. de
Groot and C.A. van Bitterswijk, "Evaluation of polylactide monomers in an in
vitro biocompatibility assay," Biomaterials, 15(4), 251-256 (1994).
21. C.F. Mandenius and L. Ljunggren, "Ellipsometric studies
of plasma protein adsorption on membrane polymers for blood purification," Biomaterials,
12, 369-373 (1991).
22. M. Spector, M.J. Michno, W.H. Smarook and G.T. Kwiatkowski,
"A high-modules polymer for porous orthopedic implants: Biomechanical compatibility
of porous implants," J. Biomed. Mater Res., 12, 665-677 (1978).
23. J.C. Knowles, G.W. Hastings, H. Ohta, S. Niwa and N. Boeree,
"Development of a degradable composite for orthopaedic use: in vivo biomechanical
and histological evaluation of two bioactive degradable composites based on
the polyhydroxybutyrate polymer," Biomaterials, 13(8), 491-496 (1992).
24. D.A. Puleo, K.E. Preston, J.B. Shaffer and R. Bizios, "Examination
of osteoblast-orthopaedic biomaterial interactions using molecular techniques,"
Biomaterials, 14(2) (1993).
25. C.R. Angle, D.J. Thomas and S.A. Swanson, "Osteotoxicity
of cadmium and lead in HOS TE 85 and ROS 17/2.8 cells: relation to metallothionein
induction and mitochondrial binding," BioMetals, 6, 179-184 (1993).
4.7 ACKNOWLEDGMENTS
We like to thank Dr. M. Heppener for active participation
in the preparations for these experiments and CCM, Nuenen, for providing the
necessary test hardware. This work is partly supported by Dienst voor de Programmatie
van het Wetenschapsbeleid (DPWB/SPPS) via PRODEX, the Space Research Organization
of the Netherlands (SRON) grant MG-004, and by the Dutch Organization of Scientific
Research (NWO) grant 900-541-133.





