Reduced Mineralization in Isolated Fetal Mouse
Long Bones Flown on Board the Russian Bion-10 Satellite.
Jack J.W.A. van Loon1, Olga Berezovska2,
Behrouz Zandieh Doulabi1, Cor M. Semeins1, Natalia V.
Rodionova2, J. Paul Veldhuijzen1.
1: Academic Centre for Dentistry Amsterdam (ACTA),
Dept. of Oral Cell Biology, Amsterdam, The Netherlands.
2: I.I. Shmalgauzen Institute of Zoology of the Academy of Sciences
of the Ukraine, Kiev, The Ukraine.
ABSTRACT
Skeletal tissues are sensitive for their mechanical environment.
Mechanical forces due to every day weight bearing ambulatory activities, are
necessary to maintain skeletal integrity. An exceptional situation of a reduced
mechanical environment is near weightlessness as a consequence of orbital spaceflight.
Several papers have indicated already that the near weightlessness
environment of space results in detrimental effects on bone matrix and/or mechanical
strength. From these in vivo studies it is not clear, however, whether
the effects are the results of a lack of load bearing on the skeleton or whether
they are, due to hormone changes or body fluid shifts as a result of entering
microgravity.
For the present study we used organ cultures of mouse long
bones as were also flown in a previous manned Space Shuttle flight. During this
unmanned Russian Cosmos-2224 mission, however, the rudiments were cultured in
completely automated tissue culture devices for a period of 4 days.
The results contribute to our earlier observations and show
that also in this completely automated experiment, fetal long bone growth was
not affected by microgravity, while matrix mineralization was decreased compared
to the 1g control conditions.
Key words: spaceflight, bone, mineralization, in
vitro.
5.1 INTRODUCTION
Future spaceflights shall be characterized by its long durations.
This is mainly due to occupation of space station Mir and the upcoming international
space station or even to missions to Mars, lasting about two years.
One of the biophysical complications of spaceflight is the
reduction of bone mass which occurs during flight. This phenomenon has already
been demonstrated in flight crews after a flight of only 7 days during Gemini(10)
as well as after the longer duration (28-84 days) SkyLab missions.(11)
More detailed studies on bone morphology and histology in rats(4)
and monkeys(13) revealed similar results of bone loss. Skeletal unloading
in the near weightlessness environment of space could be the prime possible
cause for this phenomenon. It is also possible, however, that part of the effects
reported are secondary, resulting from body fluid shifts or alterations in hormone
levels as a consequence of spaceflight.
The aim of the present in vitro microgravity experiment
was to study the effect of near weightlessness in the absence of complications
such as changes in systemic factors like hormonal levels. This experiment was
also a verification of a comparable study performed during the Space Shuttle
mission STS-42 (the first International Microgravity Laboratory, IML-1).
We have used 17 day old fetal mouse metatarsal long bones,
which were cultured in completely automated hardware on board the unmanned Russian
satellite, Bion-10, to study in vitro growth and mineralization under
microgravity conditions.
5.2 MATERIALS AND METHODS
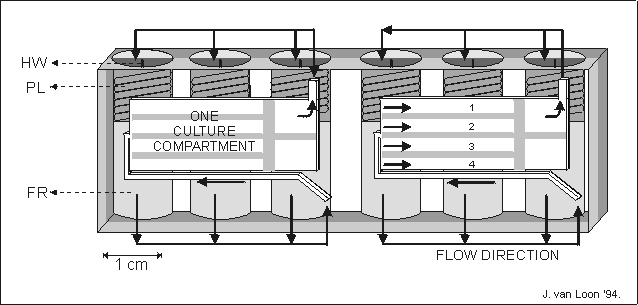
Fig. 5.1. A schematic representation of an automated tissue culture module (204080
mm (ldh)) made of a single block of polyethyleneterephthalate.
One module contains two culture compartments, 28313 mm, each
holding four metatarsal long bones, numbered 1 to 4. In a culture compartment
the long bones were separated from each other by small plates. For each culture
compartment fresh culture media of fixative were stored in three fluid reservoirs
(FR). The fluid was forces to the culture compartment by releasing a spring
loaded plunger (PL) released by scorching a nylon thread via a heat wire (HW).
The fluid was lead to the cultures via a system of internal channels and valves,
indicated by arrows. The spent medium was forced out of the culture compartment
and found its way to the, now void, volume behind the just released plunger.
The culture compartments were covered by gas permeable polyethylene foil restrained
by a perforated metal plate.
5.2.1 Tissues and Procedures
The central three cartilaginous metatarsal long bone rudiments
of 17 day old fetal (ED17) Swiss mice (University Leiden, The Netherlands) were
used. Rudiments were aseptically harvested and individually precultured in 24
wells plates, in 300 l culture medium per well in a 5% CO2
incubator for an overnight (o/n) period. The following day the metatarsals were
transferred into the hardware, the plunger boxes (PBs) (Fig. 5.1). Every PB
accommodated eight metatarsals, 4 metatarsals in each culture compartment filed
with 1.0 ml medium. After integration the PBs were handed over for transport
(see timeline). All these pre-flight preparations were carried out in the ESA
(European Space Agency) Moslab facility, at the Institute of Biomedical Problems
(IBMP) in Moscow, Russia.
By retaining the samples at room temperature (20 ± 1.5°C),
the rudiments were kept in a metabolically relative inactive state during a
100 hours lag period, used for transportation (form Moscow to Plesetsk), integration
and launch. When the spacecraft was in orbit, the temperature was increased
to 37 ± 1.5C (culture day 0). To replenish the culture medium, the first
plunger of each culture compartment was activated after 60 min in flight. A
second plunger, also releasing fresh culture medium, was activated 48 hours
later. The experiment was terminated after 96 hours by activating a third plunger,
containing formaldehyde fixative at a final concentration in the culture compartment
of 0.5% (v/v). The gas phase during the whole culture period was 5% CO2
in air.
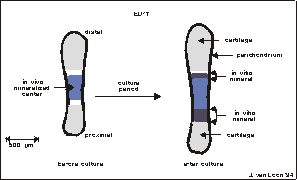
Fig. 5.2. Graphical display of a 17 day old fetal mouse metatarsal long bone
used for the Bion-10 experiment. The rudiments are ± 1.5 mm long at dissection.
Before culture, the central mineralized zone (the diaphysis) has developed in
vivo. It is flanked by zones of cartilage (the epiphysis). The whole matrix
is surrounded by a thin layer of perichondrium. During culture there is an increase
in total length as well as an increase in length of the diaphysis.
Fig. 5.3. 17 Day old mouse fetal long bone (metatarsal), after dissection
and overnight preculture. The dark center is the calcified matrix (diaphysis).
This diaphysis was mineralized in vivo, in uteri. Arrow heads represent the
edges of the mineral formed in vivo. The small protuberance at the diaphysis
is some remaining mesenchyme after dissection. Bar is 200 m.
5.2.2 Assays
A schematic drawing of a 17 day old fetal mouse metatarsal
long bone (average length at start of the experiment is 1.5 mm) is presented
in Fig. 5.2. In the 17 day old metatarsals the central part, also referred to
as the diaphysis, has already mineralized, in vivo (see also Fig. 5.3).
Under favorable conditions in vitro, these bones increase in total length
for more than 25% during 4 days culture. In parallel with this increased growth,
also mineralization advances. This can be seen in the increased length of the
diaphysis after culture (Fig. 5.3, 5.5c and 5.5d).
5.2.3 Medium
The tissue culture medium consisted of bicarbonate buffered
a-MEM without nucleosides supplemented with 50 mg/l gentamicin, 0.5% v/v fungizone
(Gibco), 0.2% BSA factor V, 3.0 mM Na--glycerophosphate (5)
(Sigma), 50 mg/l L-ascorbic acid and 300 mg/l L-glutamine (Merck). The same
medium was used for the overnight preculture in the 5% CO2 incubator
except for the addition of Na--glycerophosphate.
5.2.4 Lengths
Data on total lengths of the metatarsal long bones and the
increase length of the mineralized zone (diaphysis) were assessed from photomicrographs
taken directly before loading the samples in the PBs and again after return
of the samples in Moscow (see Figs. 5.2-5.5).
The lengths were measured from photo's which were enlarged
and digitized on a Nikon dissection microscope fitted with a Sony XC-77CE b/w
CCD camera in combination with Videoplan software (Zeiss). %Length increase
was determined as length (Lday4-Lday0)/Lday0100%.
5.2.5 Hardware and Procedures
The concept of the tissue culture modules has previously been
used for microgravity experiments.(6) The primary material used for
the tissue culture modules, or plunger boxes (PBs) was polyethyleneterephthalate
(PETP). The PBs (Fig. 5.1) were manufactured by CCM*. Before use,
the PBs were thoroughly cleaned. All PETP parts were rinsed o/n in 10% nitric
acid, changed once, followed by 24 hrs in running aqua dest, acetone for 2 hrs,
o/n in 70% ethanol, and finally air dried in a laminar flow hood. All other
PB parts were rinsed in aqua dest, ethanol 96% and air dried.
After the bones were placed in the PBs, the PBs were integrated
in CIS-boxes (Cells In Space; CCM and FSS**).(1) The CIS-box
provides a sealed gas tight environment containing all electronics for time
programmed plunger activation and housekeeping data acquisition. The CIS-box
was integrated in the Biobox (Dornier***). Biobox provides a temperature
controlled environment and supplies the interface with the spacecraft. Biobox
also accommodates a small radius, 1g reference centrifuge (radius: 71.4
mm at position of the samples). Two PBs, mounted in standard ESA Type-I containers,
were placed on the 1g in-flight centrifuge in Biobox. These two containers
were slightly modified to increase the inner volume used for the gas phase (5%
CO2 in air). The modification was to increase the gas volume inside
the Type-I containers to equal the gas volume per PB in the CIS box. After integrating
the PBs, the CIS box as well as the Type-I containers were flushed with 5% CO2
in air. Both units remained closed until after the mission, at return in Moscow.
A duplicate Biobox, with an identical configuration as the
flight model, remained on the ground. The centrifuge on this ground model contained
no biological samples. The in-fight 1g gravity field remained within
1.5% accuracy during the first 52 hours of flight. However, the speed of the
centrifuge gradually increased up to a g-level of 1.4-1.5g for the last
12 hours of the experiment.
5.2.6 Satellite
The Bion-10 or Cosmos 2224 mission was launched by a SL-4/A2
Soyuz rocket booster on December 29, 1992 at 16:30 hrs Moscow time (MT) from
the Cosmodrome launch facility in Plesetsk, Russia. After its orbital flight
the Bion spacecraft landed approximately 100 km North of Karaganda, Kazakhstan,
January 10, 1993, 07:16 hrs (MT). Recovery of the spacecraft was 12 hrs after
landing. Total flight time was 11 days, 14 hrs and 46 min.
5.2.7 Post recovery procedure
After return of the PBs in Moscow they were unloaded and the
bone rudiments were temporarily transferred to a fixative solution containing
0.5% formaldehyde, 1% sucrose in 0.1 M phosphate buffer, pH=7.6. After photomicrographs
were taken, the metatarsals were rinsed in 0.1 M phosphate buffer containing
50 mM NH4Cl and 1% sucrose, and stored in 70% ethanol during transport.
Part of the samples were processed in Amsterdam The Netherlands, the other part
in Kiev, Ukraine.
5.2.8 Microscopy
After return at the home laboratories, the samples were further
dehydrated in ethanol and embedded in either plastic (Historesin, Reichert-Jung)
or paraffin (Histoplast, Shandon). Only serial sections of 3 m were
made for light microscopic evaluation. Sections were stained with 0.2% toluidine
blue pH=4.54 for 1 min.
5.2.9 Timeline
L = launch
R = recovery
L-5 days: -Filling reservoirs with culture medium and fixative.
-Dissecting metatarsals.
-Pre-incubation of metatarsals in 24 well plates (Moscow, Russia).
- L-4 days: -Photomicrographs of samples.
-Mounting metatarsals and further integrate the PBs.
-Handover PBs to ESA officials.
-Mounting PBs in CIS-box and Biobox (20°C).
-Transport to launch site.
-Integration into the satellite.
Launch: -From Plesetsk, Russia.
L+10 min. -Start of warming up of Biobox to 37°C.
-60 min after launch, first medium change.
L+2 days: -Second medium change.
L+4 days: -Termination of experiment by fixation of the metatarsals.
L+9 days: -Lowering of the temperature in Biobox from 37 to 14°C.
R: -Recovery at Karaganda, Kazakhstan (at landing + 12 hrs).
R+2 days: -Unloading the PBs (Moscow, Russia).
-Photomicrographs of samples.
-Ethanol 70% for transport.
R+9 days: -Start histological preparations (Amsterdam, The Netherlands).
5.2.10 Statistics
After dissection the rudiments were divided into four groups.
Three groups were cultured in the specific PB hardware, namely, the actual microgravity
group (F), the in-flight 1g group cultured on the on board centrifuge
(FC) and the ground control group (G). The fourth group consisted of metatarsals
cultured in standard laboratory multiwell plates (MW). The study was divided
into two parts. One part, the F and FC groups, was flown on board the Bion-10
mission, the other part, the G and MW groups, remained on the ground.
The number of samples used for final analysis was: flight microgravity
(F) n=6, in-flight 1g (FC), n=12, ground (G), n=9 and the multiwell
group (MW), n=7.
Statistics were calculated using the two tailed Student t-test.
Data are expressed as mean ± SEM, unless indicated otherwise.
* Center for Construction and Mechatronics, Nuenen, The Netherlands)
** Dutch Space (former Fokker Space) and Systems (Leiden, The Netherlands)
*** Dornier GmbH (Friedrichshafen, Germany)
5.3 RESULTS
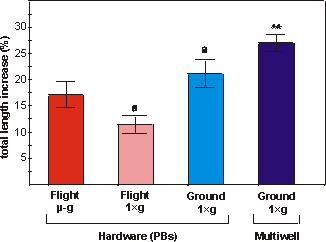
Fig. 5.4. Percentual increase in total length of bones cultured under flight
microgravity (-g, F), in-flight 1g centrifuge (FC), ground (G)
or ground multiwell (MW) conditions. Values are presented as means ± SEM. Significance
between groups is indicated by the same letter. a: p<0.005; ** the multiwell
cultures are significantly different from both flight groups (p<0.01).
A total of four groups of metatarsal long bones were used.
1: The actual flight groups; the in-flight microgravity (F). 2: The in-flight
1g control (FC). Both other groups remained on ground; one group in
plunger boxes inside a replica of the Biobox (G) and the other group was cultured
in normal 24 multiwell plates in a standard tissue culture incubator (MW).
After histological observations, it appeared that a large number
of metatarsals had not increased in length and showed a significant amount of
necrotic tissue. Based on criteria of longitudinal growth and central necrosis,
ambiguous samples were excluded. Only metatarsals which had increased in length
and showed no or only limited signs of necrosis (see Fig. 5.5e and 5.5f) were
included in the final analysis. As a result, the number of samples used for
evaluation was: flight microgravity (F), n=6 (21% of total number of samples),
in-flight 1g (FC), n=12 (100% of total number), ground (G), n=9 (56%
of total number) and the multiwell group (MW), n=7 (100% of total number).
The metatarsals showed a moderate percentual increase in length
ranging from 11.5% (FC) to 27.0% (MW) (Fig. 5.4). The increase in total length
of the microgravity group did not differ significantly from the in-flight 1g
samples (p=0.07). However, the in-flight 1g group differed significantly
from the samples grown in ground PBs (G) (p<0.005). The multiwell group increased
more in length than any other group after the 4 days culture at 37C
(Fig. 5.4).
Fig. 5.5 shows a macroscopic picture of ED17 metatarsals before
(5.5a and 5.5b) and after (5.5c and 5.5d) culture, under microgravity or on
the in-flight 1g centrifuge, respectively. A small increase in length
of the diaphysis can be seen in 5.5c as compared to 5.5a, which was characteristic
Fig. 5.5. The central part of a metatarsal long bones just
after overnight preincubation (a and b) and the same area of the same long bones
after 4 days culture in microgravity (c) or in-flight 1×g (d). 5.5e and f display
a histological section of the distal part of the metatarsal, after microgravity
and in-flight 1g, respectively. The central diaphysis is the zone with
the dark matrix at the left (D), C is the cartilaginous epiphysis and P the
perichondrium. Arrow heads represent the edges of the mineral present at dissection.
Figs. 5.5a to d, bar is 100 µm. Figs. 5.5e and 5.5f, bar is 50 µm.
for all flight microgravity samples. The increase in length
of diaphysis was less in the microgravity group compared to the in-flight 1g
group (Fig. 5.5c versus 5.5d). Histological observations of these fetal long
bones revealed no or only minor signs of cell death (Fig. 5.5e and 5.5f).
Fig. 5.6 shows that the increase in the length of the diaphysis
ranged from 76.7% (flight microgravity, F) to 221.9% (ground multiwell, MW).
Under conditions of near weightlessness there was significantly less mineral
apposition compared to the in-flight 1g group (p<0.05), this can
also be seen in Fig. 5.5c and 5.5d. Delta increase in length of diaphysis in
the in-flight 1g was also significantly different from the ground 1g
(p<0.005). In analogy with increase in total length, also the increase in
length of the mineralized zone was most pronounced in the multiwell group. Apposition
of mineral in the multiwell group was significantly increased compared to any
other group.
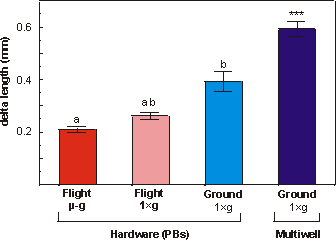
Fig. 5.6. Delta length of the diaphysis of metatarsal long bones cultured for
4 days under flight microgravity (-g), in-flight 1g centrifuge
(FC), ground (G) or multiwell (MW) conditions. Values are presented as means
± SEM. Significance between groups is indicated by the same letter. a: p<0.05;
b: p<0.005; *** the multiwell samples are significantly different from all
other groups p<0.005.
5.4 DISCUSSION
In this in vitro experiment with fetal mouse long bones,
using completely automated hardware, we studied growth and mineralization under
conditions of near weightlessness for a period of four days.
Metatarsals increased in length after a four days lag period
followed by four days culture at 37°C, but microgravity conditions had no consistent
effect on longitudinal growth. Matrix mineralization, on the other hand, was
markedly reduced in microgravity compared to in-flight 1g controls.
These data are in agreement with previous experiments performed on the Space
Shuttle as part of the first International Microgravity Laboratory Mission (IML-1).(9)
In that study the authors showed, in a different but closely related experiment,
a decrease of more then 40% in mineralization measured by 45Ca incorporation
and more than 30% in length of diaphysis in 16 day old metatarsals cultured
for four days in microgravity compared to the in-flight 1g controls.
Similar fetal mouse long bones have been used by other experimenters.(3)
They confirmed the possibility to culture metatarsal long bones under microgravity
conditions, although effects on matrix mineralization were not studied.
In ground based studies it has already been demonstrated that
mouse metatarsals are responsive to changes in gravitational forces.(7)
Growth of comparable long bones under 2.2g or 3.1g resulted
in an increased mineralization. In addition, the application of intermittent
hydrostatic pressure (an alternative way to apply mechanical loads upon these
bones) upon ED16 metatarsals also resulted in increased matrix mineralization.(2)
Both the present and previous experiments show that metatarsal long bones respond
to spaceflight, hypogravity, as well as to centrifuge, hypergravity, conditions
while in culture. Since the present study comprises an in vitro experiment,
the response of skeletal tissue to near weightlessness was not mediated by spaceflight
induced changes in levels of systemic factors such as calcium regulating hormones
or stress hormones. This implies that skeletal tissue by itself is sensitive
to decreased loads due to weightlessness as well as increased loads due to hypergravity
and hydrostatic pressure.
After morphological inspection, it appeared that a large number
of metatarsals showed a significant amount of cell death and that growth was
often impaired. For reasons unknown, this phenomenon was most pronounced in
the microgravity (F) samples. There might be several possible causes for this
phenomenon. As has been shown by van Loon et al., the biological activity
of these tissues can be slowed down for a 24 hrs period by lowering the incubation
temperature to ambient.(8) Although pilot experiments demonstrated
that bones could survive a 4 days at 20C lag period needed for transport
and integration, such treatments are not very beneficial to these tissues and
may have increased the risk of cell death. Also, one reservoir for each culture
compartment was filled with 1% formaldehyde fixative. It is possible that minute
amounts of the fixative started to contaminate the culture compartment before
the end of the incubation. This may have caused some of the metatarsals not
to increase in length and develop signs of necrosis. It is surprising that the
negative effects were absent in FC samples. The only difference in F and G compared
to FC was that the former two groups were cultured inside a CIS container, while
the latter was cultured in a slightly modified Type-I container.
Longitudinal growth as well as mineralization were more pronounced
in the multiwell control group compared to all others (Figs. 5.4 and 5.6). A
slightly diminished gas exchange in the PBs compared to the open multiwell trays
may have attributed to this difference. The PBs were covered with a gas permeable
foil while multiwell cultures were directly exposed to the 5% CO2
in air atmosphere. Also the ratio of the surface area for gas exchange compared
to the culture medium volume was less favorable in the PBs due to their mechanical
requirements and construction. Finally, the use of different materials, PETP
for the PBs and polystyrene for the multiwell plates may also have influenced
growth and mineralization. This should be tested in a separate study.
There is a significant difference in mineralization between
in-flight 1g (FC) and ground (G). A difference in calcification was
also shown between the same two groups in a previous microgravity experiment
(IML-1) using similar metatarsal long bones.(9) The differences between
in-flight 1g and ground 1g are launch accelerations and vibrations,
and in-flight cosmic radiation experienced by the in-flight 1g samples.
These factors could be responsible for the differences between in-flight 1g
and ground 1g. However, the mineralization in the in-flight 1g
was diminished in the present experiment, but augmented in the previous microgravity
study.(9) Although the flight 1g and ground 1g data
varied in the two experiments, the microgravity effect of reduced mineralization
compared to in-flight 1g remains. These discrepancies plead, again,
for the use of an on board 1g centrifuge as the best control for microgravity
experiments.
In the previous microgravity experiment(9) it was
argued by the authors that the in-flight 1g samples might have been
sensitized to the increase in gravity load during the 6 hours start-up time
between reaching orbit and the actual start of the experiment, in which microgravity
was present even in the in-flight 1g samples. Since in the Bion-10 protocol
the lag time under microgravity was only 10 minutes before starting the centrifuges,
the possibility of sensitizing seems unlikely. Also the repetitive stop and
start events, characteristic for the Biorack centrifuge during the IML-1 experiment,
were not applicable to this study. The centrifuges on board the Bion-10 operated
non-stop for the full four day period of the experiment.
Due to a software problem, the centrifuge speed of the on board
1g centrifuge began to fluctuate after the first two days in flight.
For the last two days of the experiment, the gravitational force ranged from
1.0 to approximately 1.5g (119 to 140 rpm; nominal speed 112 rpm). It
is not likely, however, that the generated increase in gravity had an influence
on growth and mineralization. It has been shown earlier that an increase in
acceleration from 1.0g to 1.3-1.5g, with comparable tissues
on a comparable centrifuge, had no impact on percentual increase in length nor
on various mineralization parameters.(9) However, greater accelerations,
from 2.2 to 3.1g, resulted in an increased growth and increased mineralization
in ED16 metatarsal long bones.(Chapter 6 and ref. 9)
In summary, in this in vitro experiment with mouse fetal
long bones, using completely automated hardware, we have demonstrated that longitudinal
growth was not affected by a near weightlessness environment in space, while
matrix mineralization was greatly reduced under microgravity. These results
confirm our hypothesis that bone mineral metabolism is affected by lack of gravity.
They suggests that the response of fetal bone to near weightlessness represents
an adaptive response to a condition of extreme unloading, which is in accordance
with Wolff's law of functional adaptation.(12) Future experiments
should focus on the cellular mechanisms by which gravity changes bone cell metabolism.
5.5 REFERENCES
1 Huijser R., Aartman L., Willemsen H. Cells In Space: sounding
rocket facilities for cell biology and biotechnology in microgravity. Proc.
Fourth Europ. Symp. Life Sci. Res. in Space, ESA SP-307, 455-466, 1990.
2 Klein-Nulend J., Veldhuijzen J.P., Burger E.H. Increased calcification of
growth plate cartilage as a result of compressive force in vitro. Arthritis
Rheum. 29, 1-9, 1986.
3 Klement B.J., Spooner B.S. Pre-metatarsal skeletal development in tissue culture
at unit- and microgravity. J. Exp. Zool. 269, 230-241, 1994.
4 Morey E.R., Baylink D.J. Inhibition of bone formation during space flight.
Science 201, 1138-1141, 1978.
5 Tenenbaum H.C. Role of organic phosphate in mineralization of bone in vitro.
J. Dent. Res. 60, 1586-1589, 1981.
6 Ubbels G.A. The role of gravity in the establishment of the dorso/ventral
axis in the amphibian embryo. In: Longdon N. David V., eds. Biorack on Spacelab
D1, ESA Publication Division, ESTEC, Noordwijk, The Netherlands, ESA SP-1091,
147-155, 1988.
7 van Loon J.J.W.A., Veldhuijzen J.P., Burger E.H. Hypergravity and bone mineralization.
ESA SP-307; Proc. 4th Europ. Symp. Life Sci. Res. in Space, Trieste,
Italy, May 1990. edt. V. David ESA Publication Division, ESTEC, Noordwijk, The
Netherlands. 393-396, 1990.
8 van Loon J.J.W.A., Veldhuijzen J.P., Windgassen E.J., Brouwer T., Wattel K.,
Vilsteren van M., Maas P. Development of tissue culture techniques and hardware
to study mineralization under microgravity conditions. Adv. Space Res., 14,
289-298, 1994.
9 van Loon J.J.W.A., Bervoets D.J., Burger E.H., Dieudonné C.S., Hagen
J.W., Semeins C.M., Zandieh Doulabi B., Veldhuijzen J.P. Decreased mineralization
and increased calcium release in isolated fetal mouse long bones under near
weightlessness. J. Bone Min. Res. 1995, accepted.
10 Vose G.P. Review of roentgenographic bone demineralization studies of the
gemini space flights. Am. J. Roentgenol., Rad. Therapy & Nuclear Med. 121,
1-4, 1974.
11 Vogel J.M., Whittle M.W. Bone mineral content changes in the Skylab astronauts.
Am. J. Roentgenol., Rad. Therapy & Nuclear Med. 126, 1296-1297, 1976.
12 Wolff J.D. Das Gezetz der Transformation der Knochen, Berlin, A. Hirschwald,
1892.
13 Zerath E., Holy X., Malouvier A., Caissard J-C., Nogues C. Rat and monkey
bone study in the biocosmos 2044 space experiment. Physiologist. 34, S194-S195,
1991.
5.6 ACKNOWLEDGMENTS
We like to thank the ESA-Moslab Bion-10 team and the IBMP-Bion-10
team for their excellent support. We also thank IBMP staff members, dr. M. Tairbekov
in particular. This research was funded by the Dutch Organization for Space
Research (SRON) grant MG-004 and the Dutch Organization of Scientific Research
(NWO) grant 900-541-133.



