7.1 GENERAL DISCUSSION AND SUMMARY
From the very beginning of the manned
spaceflight era it was speculated that the microgravity environment in space
would impose various stresses upon the human body. Besides increased cosmic
radiation and body fluid shifts, one of the hypothesized changes would be the
superfluity of the skeletons weightbearing function while in space. A relation
between mechanical loading and shape of the skeleton was already suggested by
Galilei (1564-1642).(1) Much later, in 1836, Ward(6) recognized the spatial
distribution of bone trabeculae in the human femur head and suggested this to
be related to its function of supporting mechanical loads. More then half a
century later, in 1892, Wolff published his book 'Das Gezetz der Transformation
der Knochen', postulating a 'law' saying that the shape and inner structure
of bone is a reflection of its mechanical loading history.(7) Since then many
in vivo studies have demonstrated the relation between bone structure or strength
and mechanical loading. Only few experiments have been devoted to the effect
of skeletal tissues and cells in vitro, in response to applied loads. Glucksmann,
some 50 years ago, was one of the first who demonstrated the ability of skeletal
tissues to respond to mechanical forces, while in culture.(2) Klein-Nulend et
al. applied more controllable and defined forces onto fetal skeletal tissues
in culture.(3,4) They studied the influence of mechanical forces, applied though
intermittent hydrostatic compression (ICF), on fetal mouse long bone and calvaria
development. Using this system, they showed that, in mouse long bones, hydrostatic
compression resulted in an increased mineralization and a decreased mineral
resorption compared to control, non-compressed cultures. Based on these results
we adapted this tissue culture system to be used for microgravity experiments.
Why microgravity experiments?
On Earth it is only possible to increase
mechanical stresses in tissue culture, compared to control conditions. The system
used by Klein-Nulend et al. also imposed increased stresses upon long bone rudiments.(3,4)
In spaceflight, at near weightlessness conditions, however, there are virtually
no mechanical forces acting upon matter. By culturing the fetal mouse long bones
in near weightlessness, or microgravity, in space we should see the impact of
a very low stress environment on skeletal growth and differentiation. By using
bones at two stages of development, 16 and 17 day old fetal mouse metatarsal
long bones, we may also discriminate between the two main processes in bone,
mineral formation and mineral resorption. In addition normal longitudinal growth
could be observed.
Since this is a tissue culture experiment,
it would be possible to clarify a still standing enigma resulting from the first
data on bone in association to spaceflight as discussed in Chapter 1: Is the
reduced bone density and / or strength found after spaceflight the result of
a reduced weight bearing function of the skeleton or, is this bone loss resulting
from an increased release of stress hormones or other perturbations in the systems
homeostasis as a consequence of spaceflight per se? By using the model systems
of these long bones, we could test the hypothesis that the effects seen in bones
after spaceflight are, at least partly, due to the lack of mechanical loading.
Testing a hypothesis means conducting
experiments, but especially in space research the expression 'easier said than
done' applies. After the euphoria of being offered the opportunity to conduct
a spaceflight experiment, comes a long and winding road of adapting normal,
every days’ practice or tools to 'space qualified procedures and hardware'.
When thinking of outer space and
spaceflight three things come to ones mind: weightlessness, vacuum and freezing
temperatures. The latter two phenomena were not applicable, however, for the
experiments discussed in this thesis were performed inside a spacecraft, the
interior atmosphere of which is comparable to an ambient environment. The first
property, weightlessness, or better near weightlessness (see Appendix B), is
also present inside an orbiting spacecraft and is just the reason for many researchers
to go into space. And just because of this near weightless environment, the
majority of things so common in our unit gravity world have to be redesigned
to be used in the near weightlessness or microgravity of space. Besides microgravity,
there are also the launch and time-line constrains associated with spaceflight
experiments. The hardware and the biological samples used should be such that
they can withstand the harsh launch conditions and delay in experiment initiation
times imposed upon the experimental protocols. In this respect it is surprising
that it is mandatory only for the used hardware and not the biological material,
to demonstrate that it withstands these kind of constrains.
In Chapter 2 a few of these constrains
are addressed, namely adapting standard laboratory tissue culture practice and
tools to space qualified procedures and hardware. In addition it is shown how
we could overcome the delayed initiation time of an experiment. One important
factor in tissue culture is the atmosphere in which tissues and cells grow.
Most mammalian cells need 37°C temperature and 5% CO2 in air gas phase. The
former prerequisite was provided for by the Biorack facility on board the Space
Shuttle in which the experiment had to be performed. The 5% CO2 in air gas phase,
however, was not provided by the on board facility. Only after several alternative
culture techniques, not based on a 5% CO2 atmosphere had been disqualified,
it was decided to introduce a 5% CO2 in air gas bottle for this experiment.
An other problem was the time gap between the final preparation of an experiment
and the actual initiation while in orbit. This time is needed for hand-over
procedures, transportation to launch site, integration, launch, and final start-up
while in orbit. Luckily, simply by keeping the tissues at room temperature it
was possible to overcome a 24 hours lag-period between experiment integration
and actual performance. In addition, the 24 well tissue culture plates, in which
metatarsals are normally cultured, could be replaced by polyethylene culture
bags, without changing the in vitro developmental characteristics of the rudiments.
By increasing the gas phase pressure
inside the Type-I containers the long bone rudiments are subjected to hydrostatic
stress. It has been shown previously by Klein-Nulend et al.(3) that increasing
the pressure above the culture medium produced a mechanical stress referred
to as continuous compressive force (CCF). By using mouse metatarsal long bones
it was shown that CCF had an anabolic effect on mineralization. An additional
question we also asked ourselves was that if microgravity produces effects on
fetal mouse long bones comparable to effects seen in disuse osteoporosis (decreased
mineral content), would it be possible to counteract these effects by applying
a mechanical stress through an increase of the gas phase pressure inside the
containers while under microgravity. Unfortunately, although the hardware functioned
perfectly in flight, for reasons unknown, this part of the experiment failed.
We could not duplicate the CCF effect in control cultures, hence, no conclusions
related to the hypothesis could be made.
Although hardware development is
crucial and can be an interesting part in preparing for a spaceflight experiment,
the main goal are the final microgravity results. As mentioned before, the reason
for us for going into space and experiment under microgravity conditions was
to test the hypothesis that part of the deteriorating effects found in vivo
in human and rat bone after spaceflight are due to loss of mechanical stress
applied on the skeleton. To test this, we used two slightly different in vitro
skeletal model systems. First, 16 day old (ED16) fetal mouse long bones (metatarsals)
were used in which we studied mainly matrix mineralization. In the second model,
one and a half day older, 17.5 day old (ED17.5) rudiment, we assayed osteoclastic
matrix resorption, the procedures of which are described more in detail in Chapter
3. In additions to the data on mineral metabolism, also results on growth, glucose
utilization and collagen synthesis could be obtained. By using ED16 and ED17.5
long bones for the experiment 'Bones' in the Biorack facility of ESA during
the first International Microgravity Laboratory (IML-1, STS-42, launch 22 January
1992) Shuttle flight, we have shown that microgravity indeed has an impact on
mineral metabolism in skeletal rudiments while in culture. Near weightlessness
resulted in reduced mineralization and reduced glucose utilization. In addition,
osteoclastic mineral resorption was increased. Overall growth, as measured by
increase in total length, was not or only marginally decreased under microgravity,
while collagen synthesis was not changed. All the microgravity effects were
compared to the data from the on board 1×g centrifuge samples. It is also argued
in Chapter 3 why the on board 1×g centrifuge is the best control for such spaceflight
experiments.
Part of the outcome of the IML-1
flight was verified during a Russian mission. Chapter 4 describes experiments
testing the hardware for this unmanned flight. Chapter 5 describes the setup
and results of this Cosmos-2224 mission (Bion-10) which was launched on 29 December
1992. The ED17 metatarsals used for this experiment were cultured in completely
automated tissue culture devices, so called plunger boxes. In a slightly different
experiment protocol as compared to the IML-1 setup, ED17 metatarsals were used
to study mineralization and longitudinal growth. This experiment also showed
that mineralization under microgravity conditions was decreased in the long
bone rudiments bones. In both microgravity studies, although using two completely
different sets of hardware and timeline constrains, fetal mouse metatarsal long
bones reacted with a significantly decreased matrix mineralization to the near
weightlessness environment.
While preparing for the Russian unmanned
Bion-10 flight, some modifications to the hardware had to be made, which are
described in Chapter 4. Special tissue culture devices, already developed for
earlier spaceflight applications, had to be adapted to our needs. Some engineering
had to be performed in addition to biological testing. It appeared that the
initially chosen material, polysulphone (PSU), did not allow mineralization
of bone rudiments as well as bone marrow cultures, which were planned to be
used for the spaceflight study. Both studies at the Flemish Institute for Technological
Research (VITO) in Belgium, as well as in our laboratory showed the inability
of skeletal tissues and cells to differentiate in contact with PSU leacheble
factors. Typical markers in bone growth and development like alkaline phosphatase,
collagen formation and matrix mineralization were strongly reduced or completely
absent in cultures with polysulphone plastics. Surprisingly, there was no effect
on proliferation, measured by 3H-thymidine incorporation into osteoblast like
cells or increase in total length of metatarsal long bones. Eventually, instead
of polysulphone, polyethyleneterephthalate (PETP) was chosen as prime material
for the construction of the tissue culture module to be used for the Bion-10
experiment.
Polysulphone polymers are also used
in orthopedic implants. Therefore the experiments prepared for this spaceflight
experiment appeared of interest for the orthopedic practice. The results of
the biocompatibility tests argue against the use of polysulphone in contact
with bony tissue, as used in bone replacement therapy.
To complement experiments performed
under microgravity, Chapter 6 describes experiments performed with fetal mouse
long bones cultured at increased gravitational stresses. Under increased accelerations,
or hypergravity, growth and mineral metabolism were studied in ED16 and ED17
metatarsals. Most of the experiments were performed using a self-made prototype
tissue culture centrifuge, while for the pilot experiments performed at the
University of Texas in Houston, a more advanced tissue culture centrifuge was
used.
In both experimental setups it was
demonstrated that increased gravity ranging from 2.2 to 3.1×g resulted in increased
matrix mineralization in ED16 metatarsals. Total length, as an indicator for
general growth, was also increased in 2.2×g ED16 bones compared to 1.0×g controls.
17 Day old 45Ca prelabeled rudiments, used for mineral removal studies, showed
that under 2.2×g calcium release was increased, indicating increased osteoclastic
activity.
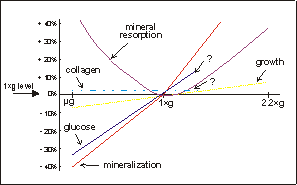
Fig. 7.1. Schematic representation of various parameters of skeletal growth
and differentiation, the data of which is based on the experiments described
in this thesis. Under microgravity there is a decrease in glucose utilization
and mineralization, and probably also retarded longitudinal growth. Mineral
resorption is increased under microgravity. Under hypergravity conditions, however,
longitudinal growth is increased and there is an augmented mineral resorption,
compared to 1×g controls.
The results of Chapter 3,5, and 6,
may be combined to show the effects of microgravity and access gravity on fetal
mouse long bone development. Fig. 7.1 shows a schematic representation of the
microgravity and hypergravity results. Mineralization and growth show a continuum
in response to gravity through the 1×g reference point. They are both decreased
under microgravity and augmented under increased gravity. This in contrast to
osteoclastic mineral resorption, which is increased both under micro- as well
as hypergravity conditions. For collagen synthesis and glucose utilization only
two values are available, microgravity and 1×g. Hypergravity experiments are
needed to extend these lines.
The non-linear response on mineral
resorption could mean that there are two different processes involved in this
response. Under microgravity mineral resorption may be increased due to lack
of mechanical forces, which causes bone loss. In case of hypergravity, the increase
in mineral resorption may be a result of a continuous stress applied onto these
fetal mouse long bones.
Parameters like glucose utilization
and collagen synthesis still have to be measured in hypergravity cultures. In
order to facilitate future hypergravity experiments a new centrifuge has been
build. A study by van Loon et al. describes the requirement study and subsequent
construction of the Medium sized Centrifuge for Acceleration Research (MidiCAR).(Appendix
A and ref. 5) The realization of this project was a close collaboration of various
gravitational scientists, industry, and the national Dutch Organization for
Space Research (SRON). This apparatus provides future experimenters with a well
controlled and convenient apparatus to conduct hypergravity experiments. Although
the results of these hypergravity studies do not necessarily have to be mirror
images of microgravity data (see Fig. 7.1), they contribute to our knowledge
of cellular processes in relation to accelerations i.e. mechanical forces, applied
upon skeletal as well as various other tissues and cells. On the other hand,
the microgravity environment provides a unique place to survey the effects of
nearly zero mechanical force on fetal bone growth and differentiation in vitro.
In this way both microgravity as well as hypergravity experiments contribute
to the identification of the mechanotransduction system in skeletal cells and
tissues. Hence these gravitational studies indeed contribute to our understanding
of Wolffs' law at the cell- and molecular level.
Under microgravity several physical
phenomena are changed compared to on Earth 1×g or centrifuge hypergravity conditions
(Appendix B). Surprisingly, still very little is known, even within the field
of gravitational science, about the impact of various physical phenomena on
biological systems. It has been documented for a long time already that events
like convection, hydrostatic pressure or Coriolis forces are influenced by accelerations,
i.e. gravity. Several experiments have now been performed on the impact of reduced
gravity in cells and tissues, but usually these studies have not addressed the
question of primary or secondary effects of gravity. It should be realized that
effects seen in a spaceflight experiment may be indirect resulting from changes
in the tissues' environmental conditions, in contrast to direct effects of microgravity
on cells. Therefore, Appendix B provides a brief introduction to physical phenomena
which can be of value to microgravity of hypergravity experimenters. Future
experiments could systematically screen the impact of these processes in biological
systems. Most of these experiments can be done on Earth with occasional checks
under microgravity conditions. Only than, direct (micro-)gravity effects can
be distinguished from secondary, environmental causes. These questions urge
for further cooperation and integration of the two disciplines of biology and
physics. Spaceflight experimentation in particular provides common grounds for
both kinds of science and this could be synergistic for future understanding
of the behavior of cells in space, the field of gravitational biophysics.
7.2 CONCLUSION
In conclusion this thesis reports,
for the first time, a comprehensive study of the effect of various accelerations,
ranging from near weightlessness to an access gravity of more than 3×g on mineral
metabolism on bone organ cultures. The bone rudiments respond to the lack of
gravity by a decreased mineralization and glucose utilization, and increased
mineral resorption, while general growth and collagen synthesis are, statistically,
not affected. Increasing accelerations to 2.2×g, result in increased growth,
increased mineralization as well as increased mineral resorption. Decreasing
the mechanical load leads to decreased bone mineral content while increasing
the stress resulted in increased mineralization. These results show that the
ideas of Wolff also pertain to fetal mouse long bones cultured under various
gravity conditions.
Future ground based hypergravity
in conjunction with spaceflight hypogravity experiments, should be devoted to
the search for the mechanosensor system in skeletal tissues and cells. More
research is needed in the area of second messengers, signal transduction, stretch
activated ion channels, membrane potentials and the role of the cytoskeleton.
In the long run, studies on the cellular behavior in response to mechanical
stresses contribute to the knowledge and understanding of the pathogenesis and
possible treatment of immobilization osteoporosis and, maybe, other biomechanical
related disorders.
7.3 REFERENCES
1 Galileo G. Two new sciences. In;
"The second day". Translated by Stillman Drake, The University of Wisconsin
Press 109-146, 1974.
2 Glucksmann A. Studies on bone mechanics in vitro. II. The role of tension
and pressure in chondrogenesis. Anat. Rec. 73, 39-56, 1942.
3 Klein Nulend J., Veldhuijzen J.P., Burger E.H. Increased calcification of
growth plate cartilage as a result of compressive force in vitro. Arthritis
Rheum. 29, 1-9, 1986.
4 Klein Nulend J., Veldhuijzen J.P., Strien M.E. van, Jong M. de, Burger E.H.
Inhibition of osteoclast bone resorption by mechanical stimulation in vitro.
Arthritis Rheum. 33, 66-72, 1990.
5 van Loon J.J.W.A., van den Bergh L.C., Schelling R., Veldhijzen J.P., Huijser
R.H. Development of a centrifuge for acceleration research in cell and developmental
biology. 44th International Astronautical Congress, IAF/IAA-93-G.4-166, 39,
Graz, Austria, 16-22 October 1993.
6 Ward F.O. Outlines of human osteology. 3rd edition, Henry Renshaw, London,
1836.
7 Wolff J. Das Gezetz der Transformation der Knochen, Berlin, 1892.
