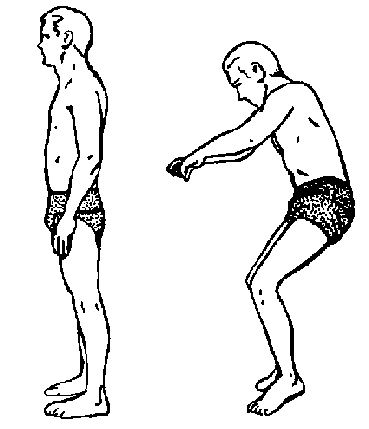
Figure 1: Relaxed erect body posture. Relaxed
erect body posture on earth (left) and under weightlessness (right). From:
Wichman & Donaldson, 1996.
Chapter 2: The disadvantageous physiological effects of spaceflight
Spaceflight is accompanied by several disadvantageous effects on various physiological systems. The lack of gravitational forces, the confinement in a relatively small spacecraft, and the increased exposure to radiation elicits disturbances to the musculo-skeletal, cardiovascular, vestibular, pulmonary, thermoregulatory, immune, metabolic, and reproductive systems (Tipton, 1983a; Bachl et al., 1993; Lane et al., 1993; Tipton & Hargens, 1996). A lot of these disturbances are related to each other.
In this chapter, the expected changes during long-term spaceflight in all of these systems will be discussed. The main interest will be on the changes of the musculo-skeletal system, as this is expected to be the limiting factor during future long-term missions (Bachl et al., 1993). Paragraph 2.1 will focus on muscle tissue, first presenting the basic muscle physiology and then presenting the muscle related problems during spaceflight. Paragraph 2.2 will have an identical structure, now focusing on bone tissue. Possible countermeasures, with the main interest on exercise, and the exact mechanisms behind the changes in muscle tissue and bone tissue will be discussed in chapter 3 and chapter 4 respectively. The remainder of this chapter will present the spaceflight-induced changes on the other physiological systems mentioned above, primarily focusing on the effects of future long-term missions.
2.1 - The effects of spaceflight on muscle tissue
2.1.1 - The physiology of muscle tissue
In the human body, several types of muscle tissue are present. The type that is mainly affected by spaceflight is the type of muscle directly attached to the skeleton, i.e. skeletal muscles. The organization of skeletal muscles is thoroughly described in the literature (e.g. Jones & Round, 1992). In this paragraph, the relevant basic knowledge about skeletal muscles will be outlined, so the changes that occur during spaceflight can be understood better. Additionally, certain characteristics of skeletal muscle should be borne in mind in order to be able to determine changes in muscle strength correctly. In this literature review, skeletal muscle will be addressed as "muscle".
Muscles are made up by fibers. These fibers contain myofibrils, which are filled with myofilaments. Myofilaments mainly contain two proteins: actin and myosin. Approximately 80 per cent of the total protein content of skeletal muscles exist of actin and myosin. These two proteins interact with each other to produce muscle contractions. Other proteins that are involved in muscle contraction are troponin, tropomyosin and titin (Jones & Round, 1992). The layered structure of the muscle ensures that when actin and myosin start to contract in a controlled manner, this contraction, and thus the strength that is delivered, can take place throughout the whole muscle. Contractions take place at the cost of adenosine triphosphate (ATP), which is the energy source of all contractions (Rozendal et al., 1990; Jones & Round, 1992).
Based on itís contractile properties, muscle fibers can generally be classified into three types. Type I, or slow oxidative fibers, are characterized by a relatively slow development of force but are able to sustain this force relatively long. Type IIa and type IIb fibers are able to develop force faster. Type IIb, or fast glycolytic fibers, has the fastest properties, at the cost of lowered endurance. Type IIa, or fast oxidative glycolytic fibers, are intermediate fibers (Gordon & Pattullo, 1993; Leterme & Falempin, 1994). The type of muscle fiber can be determined by studying the heavy chains of the myosin molecule or by studying troponin or myosin light chains (Edgerton et al., 1995; Zhou et al., 1995).
The classification of fibers into types I, IIa and IIb is based on the ATP-ase activity of the fiber, while the classification into oxidative and glycolytic fibers is based on the metabolic enzymes that are involved (Gordon & Pattullo, 1993). Co-expression of two fiber types within one fiber is possible (Baldwin, 1996). Alterations in loading patterns, like chronic stimulation or disuse, can bring about changes in this co-expression. The disuse of muscles during spaceflight, for example, induces a shift from slow to fast, which means that fast myosin and fast troponin isoforms become present in slow muscle fibers. Changes can occur within eleven days of spaceflight, and have also been found after specific training regimens (Roy et al., 1991; Zhou et al., 1995). However, a fiber always continues to express itís original heavy and light chains (Gordon & Pattullo, 1993; Zhou et al., 1995).
The development of force by a muscle is dependent on the length of the muscle and on the velocity of contraction. The alignment of myosin and actin ensures a specific length of the muscle at which optimal force development can take place. If the muscle is shorter or longer, the alignment of myosin and actin becomes less optimal and less force can be produced. Because of this so-called force-length relationship it is very important to standardize the angles of the relevant joints (i.e. standardization of the length of the muscle) when comparing muscle strength production before and after a certain period of time. Obviously, in bi-articular muscles, both joints should be in a standardized angle (Rafolt & Gallasch, 1996).
Similar to the strict force-length relationship of a muscle, there is a strict force-velocity relationship in muscle contractions. The highest forces can be developed at slower velocities of contraction, as there is a more optimal relationship between actin-myosin coupling and de-coupling at those velocities. Higher velocities make this relationship less optimal, up to a point where maximum velocity is reached, and force production is zero (Rozendal et al., 1990; Jones & Round, 1992). Consequently, it is also important to compare muscle strength production at identical angular velocities (i.e. standardization of the velocity of contraction).
The power a muscle can generate is largely dependent on the amount of actin-myosin filaments that can be used. More filaments means more potential to generate muscular pull. The length and size of a muscle fiber can vary considerably between various muscles in the body and between individuals of different sex, build, and age. The length of a muscle fiber can vary between several millimeters and approximately fifteen centimeters (Rozendal et al., 1990; Jones & Round, 1992), and is mainly responsible for the maximum velocity of contraction (Rozendal et al., 1990). The strength of a muscle is mainly determined by the size of myofilaments, which is often indicated by the surface area of a perpendicular slice of the muscle, the cross-sectional area (CSA; Rozendal et al., 1990). The interdependence between muscle mass and muscle strength is visible in the high correlations found between maximal strength and CSA of a specific muscle (Dudley et al., 1992; Edgerton et al., 1995). CSAs vary between 2500 m m2 and 7500 m m2 (Jones & Round, 1992). Training increases the size of muscle fibers and perhaps even the number of muscle fibers, thereby increasing the maximal strength (ACSM, 1990).
Muscle contractions are often referred to as isometric, isotonic or isokinetic. These terms refer to contractions that are associated with respectively constant fiber length, constant tension, and constant shortening velocity. An example of an isometric contraction is pushing against an immovable wall. Although the muscle does not shorten (i.e. the velocity is zero), this still costs ATP because of the continuous generation of force. Since there is no visible movement, this is called a static contraction, as opposed to dynamic contractions. True isotonic contractions do not exist, because the arm of the force that is generated in the muscle changes while the muscle shortens. Therefore, the generated torque, or tension, changes as well. Isokinetic contractions are seldomly seen in daily life activities, but are the main object of research in studies focusing on muscular strength, because of the strict force-velocity relationship of every muscle.
Another classification of muscle contractions, is into isometric, concentric, or eccentric contractions. Concentric contractions means that the muscle fibers decrease in length. Under the influence of external forces, muscle fibers can increase in length while contracting. This is called an eccentric contraction. An example of an eccentric contraction is walking downstairs, when the force of gravity causes the muscle to lengthen while contracting. During eccentric contractions, the force that is produced by the muscle is even greater than during isometric contractions. This greater production of force is still unexplained, but is surprisingly at the cost of hardly any ATP (Rozendal et al., 1990; Jones & Round, 1992). When gravity is absent, eccentric contractions rarely occur, which has been suggested to be an important reason why muscles atrophy in microgravity (Convertino, 1991; Kirby et al., 1992; Hortobagyi et al., 1996).
The force, velocity and fatigue characteristics of a muscle are determined by the myosin and troponin isoforms and by the oxidative and glycolytic enzymes (Roy et al., 1991). Besides these biochemical properties, neural aspects are also important in the determination of the characteristics of a muscle. Neural control of muscle contractions progresses by motor units. One motor unit consists of a motoneuron and an indefinite number of muscle fibers, scattered around the entire muscle (Jones & Round, 1992). It is important to recognize these neural aspects of muscle contractions, since problems on the contractile level can be either due to neural or due to muscular problems. Muscular co-ordination, such as optimal neural co-operation of the agonists and antagonists and optimal recruitment patterns of the motor units, has a large impact on the maximal force that can be generated.
2.1.2 - Muscle mass decrements due to spaceflight
During spaceflight both muscle mass and muscle strength are reduced. The reduction in muscle mass, or muscle atrophy, is synonymous to a reduction in contractile elements. However, the number of muscle fibers is not decreased during spaceflight (Stein & Gaprindashvili, 1994). The exact sequence of the loss in muscle mass is not exactly clear. Both protein synthesis and protein degradation processes are altered in bed rest studies (Baldwin, 1996). During spaceflight the reduction in protein synthesis seems to be the primary cause for the reduction in muscle mass (Stein & Gaprindashvili, 1994).
In vivo measurements of muscle mass is possible with modern techniques, such as dual-photon absorptiometry and magnetic resonance imaging (LeBlanc et al., 1992). However, no spaceflight data have been reported with the use of these techniques. Therefore, only estimations, based on indicators of muscle breakdown, are available to quantify the spaceflight-induced muscle mass decrements.
A possible indicator of the reduction in muscle mass is the loss of nitrogen during spaceflight or one of itís terrestrial simulations. Nitrogen is an essential element of every protein. Skeletal muscle is the largest active protein pool in the body, thereby being the major site of protein loss (Stein & Gaprindashvili, 1994; Bloomfield, 1997). Thus, the determination of nitrogen excretion in urine is an indicator of muscle tissue breakdown. This concept was confirmed by the finding of increased excretion of the proteins 3-methylhistidine, creatinine, and sarcosine during spaceflight, which indicates muscle breakdown (LeBlanc et al., 1992; Stein & Gaprindashvili, 1994).
The visible "shrinking" of, mainly, the legs has also been used as an indicator of muscle atrophy. After approximately six months of spaceflight, the circumference of the legs has decreased by several centimeters (Convertino, 1990; Grigoriev et al., 1991b; Stein & Gaprindashvili, 1994). Although the loss of muscle mass is likely to be partially responsible for this decrease, this shrinking is also influenced by a cranially directed fluid shift, which is caused by microgravity (this shift will be discussed in paragraph 2.3.1). This fluid shift causes a loss of fluids in the legs. Consequently, this measure is a less reliable indicator of muscle atrophy.
Spaceflight-induced muscle atrophy is caused by the relative disuse of the muscles in a microgravity environment (Stein & Gaprindashvili, 1994; Convertino, 1996; Convertino et al., 1997). The lack of gravitational pull, which is the main external factor influencing the muscle under terrestrial conditions, requires a lessened use of the muscle. Also, especially in the early years of spaceflight, the confinement in a small spacecraft did not allow for a lot of movement during the flight. Four major factors largely influence the degree of muscle atrophy: the function of a muscle on earth, flight duration, food intake, and the amount of exercises performed in-flight.
Muscle atrophy is influenced by the function of a muscle on earth. The degree of atrophy is different for various muscles. The muscles of the arms and shoulders show smaller losses than the muscles of the lower back, abdomen, thighs and lower legs (Hayes et al., 1992; McBrine et al., 1992). These lower body muscles are critical to the maintenance of posture and balance on earth (Bloomfield, 1997). Hence, these muscles, also called postural muscles or weight-bearing muscles, suffer the most from the disappearance of gravity (Vorobyov et al., 1983; Stein & Gaprindashvili, 1994). The smaller losses in the upper limb may also be caused by an increased use of the arms during spaceflight. Under weightlessness, predominantly the arms are used to manoeuvre within the spacecraft and during extra-vehicular activities (Bloomfield, 1997).
Flight duration is also an important determinant of muscle atrophy. In the first week of weightlessness, atrophy is still absent in most humans (Convertino, 1990), but after eleven days the loss has become significant in a population of five astronauts (Edgerton et al., 1995). Although it is suggested that, in rats, the decrease in soleus muscle mass may plateau after two weeks of spaceflight (Edgerton et al., 1995), it is likely that in humans the loss in muscle mass will continue to occur throughout the duration of spaceflight. It is believed that after a substantial loss in the first four months of weightlessness, muscle atrophy will continue at a slower rate (Tipton et al., 1996).
A third factor that influences the degree of muscle atrophy is food intake. It is likely that cosmonauts and astronauts are to some degree in an energy-deficit state during spaceflight, with the consequence that muscle protein will be lost (Stein & Gaprindashvili, 1994). This problem becomes less important with increasing mission length, as in longer missions crew members have more time to prepare and consume food and consequently get closer to the recommended daily energy intake (Lane et al., 1994).
A major influence is the amount of exercise performed in-flight (Bachl et al., 1996). Exercise has the ability to increase muscle mass on earth, and is also useful in minimizing losses in muscle mass during periods of disuse, such as spaceflight (Jones & Round, 1992; Convertino, 1996; Bloomfield, 1997). The performance of in-flight is very seldom entirely quantified (Convertino, 1990), making it very difficult to make any statements about the effects of exercise during a spaceflight. The goal of in-flight exercises is not as much to increase muscle mass per se, but to increase muscle function, i.e. muscle strength.
2.1.3 - Muscle strength decrements due to spaceflight
Muscle strength is mostly defined as the maximally achievable torque during a specific contraction. Spaceflight generally causes a decrease in muscle strength. Significant decreases in strength of the trunk, knee and shoulder have been found within six days of a stay in microgravity (Convertino, 1990; Hayes et al., 1992). Some data with respect to the changes in muscle strength are shown in table 2.
Table 2: Muscle strength changes in humans after spaceflight. Spaceflights are listed in chronological order. All changes are presented as percentages (averaged over the number of crewmembers) as compared to pre-flight muscle strength.
|
Reference |
N1 |
Muscle or muscle- |
Angular velocity (ø /s) |
|||||
|
0 |
45 |
60 |
120 |
180 |
Not reported |
|||
|
Convertino, 1990 |
12 |
back strength |
-15 |
|||||
|
Greenleaf et al., 1989 |
9 |
elbow flexors |
-4 |
|||||
|
Kozlovskaya et al., 1990 |
3 |
m. gastrocnemius |
-35 |
-28 |
-30 |
-24 |
||
|
Kozlovskaya et al., 1990 |
2 |
m. gastrocnemius |
-47 |
-1 |
+25 |
+19 |
||
|
Grigoriev et al., 1991b |
2 |
m. tibialis anterior |
range: |
|||||
|
Edgerton et al., 1995 |
2 |
knee extensors |
-50 |
-20 |
-10 |
|||
1
N = number of crewmembers.
2 The orbiter Mir does not use sequential numbers. However,
these are values of the second crew attending.
3 These are data of the third crew attending the Mir.
It would be expected that the decrements in muscle strength resemble the decrements
in muscle mass. Thus, larger losses in the postural muscles and larger losses
with increased flight duration would be expected. The amount of data in table
2, however, is not large enough to confirm these expected relationships. It
is surprising to see that, while the spaceflight-induced decreases in muscle
strength are so commonly accepted, so few exact data have been made available
to the scientific community.
It is usually stated that isometric strength (i.e. maximal muscle contraction
at an angular velocity of 0 į/s) is affected most, with faster speeds of contraction
showing smaller losses in strength (Convertino, 1990; Edgerton et al., 1995).
The few strength data that are reported at several isokinetic speeds do support
this statement.
The gastrocnemius muscle strength data at 120 į/s and 180 į/s in the third crew
attending the Mir are anomalous. Increases in strength are nowhere else reported.
No explanation was given for these findings by the researchers (Kozlovskaya
et al., 1990). Convertino hinted that dynamic calf strength during long-term
flights might be used more intensely (Convertino, 1990). However, since the
second crew attending the Mir showed large decrements, this could indicate that
the countermeasures performed by the third crew attending the Mir were very
effective.
Figure 1: Relaxed erect body posture. Relaxed erect body posture on earth (left) and under weightlessness (right). From: Wichman & Donaldson, 1996.
The decrease in muscle strength is commonly more profound in the extensor than
in the flexor muscle groups in the legs (Tesch et al., 1990; Dudley et al.,
1992; Bachl et al., 1996). This may be due to the posture of humans in microgravity.
In space, comfortable posture is somewhat altered, placing the feet in an extended
position (figure 1). This stretches the dorsal flexor muscles (also known as
ankle flexor muscles), such as the tibialis anterior, thereby altering the electrostimulation
of these muscles. This may influence the degree of atrophy, maintaining size
and strength of the stretched muscles (Dudley et al., 1992; Gordon & Pattullo,
1993). However, table 1 does not support this statement. In the third Mir crew
the tibialis anterior muscle showed greater losses than itís antagonist, the
gastrocnemius muscle, and in the Skylab crews the knee flexor and extensor muscles
showed similar losses.
Apparently, the common statements made in the literature about the decrements
in muscle strength do not completely agree with the data made available in scientific
journals, as presented in table 2. This is largely due to the small number of
data that could be presented in table 2. Unfortunately, no more adequately described
spaceflight-related muscle strength data were made available to the scientific
community. This is a common problem in space-physiology (Convertino, 1990; LeBlanc
et al., 1990). Further aspects that might confound the data in table 2 are the
unknown influences of the unreported nutritional status and the unreported performance
of in-flight exercises by the crewmembers. Consequently, much remains unknown
about the changes in muscle strength during spaceflight. However, during spaceflight,
losses in muscle strength are expected due to the disuse of muscles during flight.
The occasionally found increases in muscle strength might indicate that an effective
countermeasure protocol is possible.
Besides losses in muscle mass and muscle
strength, losses have also been found in muscular stamina and contractile endurance,
both in humans and in rats (Baldwin, 1996). Again, these findings were made
in the legs, but not in the arms (Convertino, 1990). Although the underlying
mechanism may be different, the reasons for these losses are thought to be identical
to the reasons for maximal strength losses: disuse due to unloading and confinement
in a small space.
2.1.4 Effects of long-term spaceflight on muscle tissue
The losses in muscle mass and muscle strength may not yet have been detrimental
to work and exercise performance during spaceflight, but in future long-term
flights they could become the most important limiting factor (Bachl et al.,
1993). Muscle atrophy is likely to continue throughout spaceflight (Tipton et
al., 1996), and is thus a major concern for future missions of approximately
three years of duration.
In addition to the in-flight muscle problems, the losses in muscle mass and
muscle strength could be a significant limiting factor upon return to 1G following
prolonged spaceflight and could limit spacetravellers in their activities thereafter
(Convertino, 1990). In case of an emergency regress during or directly after
landing, spacetravellers must be in sufficient shape to be able to regress without
any help from others.
2.2 - The effects of spaceflight on bone tissue
2.2.1 - The physiology of bone tissue
Human bone consists of collagen matrix, which gives the bone a certain degree
of elasticity. The matrix has a labyrinth-like structure, the cavities of which
are filled with mineral salts, mainly calcium hydroxyapatite (3Ca3(PO4)2ù
Ca(OH)2; Mack et
al., 1967). These minerals give bone itís strength. An estimated 80 per cent
of bone-strength is determined by pure bone mass. The remaining 20 per cent
is determined by the labyrinth-like structure of bone (Bosch, 1993).
There are two distinguishable structures of bone. Cortical bone, which is very
compact and dense, forms the outer layer of a bone. More inward the bone becomes
more spongy with a network of multiple thin cross-links. This is called trabecular
or cancellous bone (Snow-Harter & Marcus, 1991; Chilibeck et al., 1995).
On average, the ratio of cortical to trabecular bone over the whole body is
4:1, but this ratio can differ substantially between different bones. Long bones,
like the proximal femur, have a ratio of approximately 9:1, while the ratio
in lumbar vertebrae is 1:2 (Chestnut, 1987).
Bone is continually being remodelled under the influence of three types of cells,
each with itís own functions. Firstly, osteoblasts, or bone forming cells, synthesize
the collagen matrix and control the mineralization of the bone. Secondly, osteoclasts,
or bone resorption cells, secrete acids, which dissolve the minerals and act
against the formation of bone-components. Finally, osteocytes preserve the homeostasis
of bone formation and resorption. Osteocytes are differentiated osteoblasts,
which have become active to form bone, and are capable of both synthesis and
resorption. (Snow-Harter & Marcus, 1991).
Bone-remodelling is a continuous process throughout life. An, as yet, unknown
trigger activates the osteoclasts to form holes and tunnels. These holes and
tunnels are filled with new matrix by the osteoblasts. Twelve to fifteen days
after this, the mineralization of the newly formed bone starts. Complete mineralization
has taken place after six to twelve months (Chilibeck et al., 1995).
The process of bone-remodelling is different for the two types of bone. The
loss of trabecular bone starts earlier and is of a higher rate compared to cortical
bone (Snow-Harter & Marcus, 1991). This is caused by the structure of trabecular
bone: remodelling takes place primarily on the surface area of bone, and the
network structure of trabecular bone causes the surface area to be much larger
than that of itís cortical counterpart. This has implications for which bones
are at risk at higher age. Because of their odd bone structure, the lumbar vertebrae
are more prone to osteoporotic fractures. Trabecular bone is also more affected
by spaceflight induced osteoporosis (Schoutens et al., 1989; Zernicke et al.,
1990; Convertino, 1996).
The places where these processes take place are called remodelling units. There
are over one million remodelling units in a healthy skeleton. In adults, 20
to 30 % of the bone is replaced each year (Bosch, 1993). The balance of the
osteoclast and osteoblast activity is not even. Until approximately the age
of thirty, more bone is being formed than there is being dissolved, with an
extra strong positive balance during puberty. Thereafter, the balance becomes
negative and the total amount of bone decreases (Welten et al., 1994; Botden
& Kemper, 1996). The age when peak bone mass is reached is called skeletal
maturity. The loss of bone starts later in males compared to females due to
hormonal differences. During menopause the rate of bone loss is strongly increased.
Therefore, females are more at risk of osteoporosis than males (Botden &
Kemper, 1996).
This loss of bone mineral after the age of thirty should be borne in mind. The
oldest male astronaut to have flown in space was 56 years old (Convertino, 1996).
Under terrestrial conditions, a loss in bone mineral density (or BMD) of 0.5
to 1 percent per year would be considered normal at such age (Swezey, 1996),
so losses in a microgravity environment cannot solely be attributed to that
environment.
2.2.2 - Bone mineral losses due to spaceflight
Some reported values of bone losses after actual spaceflight are presented in
table 3. It should be noted that the crew of the Apollo XV spend 67 hours of
their journey on the moon, which gravity is one sixth of that of the earth (Wickman
& Luna, 1996). However, their calcaneal bone mineral density losses indicate
that such a stay does not appear to have a substantial bone sparing effect.
To assess bone mineral density (BMD) various densitometric techniques can be
used. In table 3 the data from the Gemini and Salyut I flights have been determined
by X-ray densitometry; the Apollo XV, Skylab and Salyut VI data by single photon
absorptiometry (SPA); the cosmonaut of the third crew attending "Mir" was examined
with computer tomography (CT) and all other crew members in the "Mir" with dual
energy photon absorptiometry (DPA). Of these techniques, only CT gives a true
density (i.e. volumetric, in g/cm3); all other techniques give areal
densities (in g/cm2), and are thus mainly reflections of cortical
bone density (Snow-Harter & Marcus, 1991; Prentice et al., 1994). All techniques
have their own different advantages and disadvantages, but are accurate within
a five percent range and have a satisfactory reproducibility, if used correctly
(Chestnut, 1987).
Table 3: Changes in bone mineral density in humans after spaceflight. Spaceflights
are listed in chronological order. All changes are presented as percentages
(averaged over the number of crewmembers) as compared to pre-flight bone mineral
density values.
|
Reference |
N1 |
Hand |
Spine |
Thigh |
Foot |
|||||||
|
Orbiter (days in orbit) |
Capi-tate |
Phalanx 4-2 |
Phalanx 5-2 |
L1,L2, |
Tro. |
Cap. |
Talus |
Calca-neus |
||||
|
Mack et al., 1967 |
|
|
|
|
|
|
|
|
|
|||
|
Rambaut & Goode, 1985 |
|
|
|
|
|
|
|
|
|
|||
|
Rambaut & Goode, 1985 |
|
|
|
|
|
|
|
|
|
|||
|
Tilton et al., 1980 |
|
|
|
|
|
|
|
|
|
|||
|
Rambaut & Goode, 1985 |
|
|
|
|
|
|
|
|
|
|||
|
Grigoriev et al., 1991b |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||||
1 N = number of crewmembers.
2 Tro. maj. = Trochanter major.
3 Cap. fem. = Caput femoris.
4 The orbiter Mir does not use sequential numbers. However, this value is of one cosmonaut of the third crew attending.
5 These are combined data of the sixth to ninth crew attending the Mir.
* These are five years post-flight values.
It is generally believed that the legs, the pelvis area and the lumbar vertebrae show the highest losses (Oganov et al., 1992; Tipton & Hargens, 1996). On earth, these bones are subjected to the effects of gravitational pull in a 1G environment, and are concomitantly called "weight-bearing bones". Hence, these bones are most prone to suffer from the disappearance of gravitational forces (Zernicke et al., 1990).
Table 3 does not actually support this statement, also showing major losses in the hand. This disagreement can be attributed to several confounding influences that are linked to bone metabolism. The three major confounding influences are the duration of the stay in a near weightless environment, the nutritional status of the flight members (especially calcium intake), and the in-flight performance of exercise.
Firstly, the duration of a stay in weightlessness is a major determinant of the observed decrements in BMD. The rate of loss amounts approximately one half to two per cent per month, depending on gender and anatomical site (Tipton & Hargens, 1996). Although some Russian scientists have suggested that bone demineralization may plateau, these losses are likely to continue throughout the total duration of the flight (Zernicke et al., 1990).
The main mineral in bone is calcium, which makes calcium intake an important determinant of bone mineral density. During flight, calcium intake not exceeding 950 mg/day has been shown to correlate significantly with bone loss (Mack et al., 1967). However, this relationship is not continuously linear. Any calcium consumption above 1200 mg/day is not believed to have extra bone sparing effects (Snow-Harter & Marcus, 1991). The surplus of calcium is immediately excreted. Lane et al. (1994) state that 800 mg a day is already sufficient. These researchers agree that sufficient calcium intake is an important condition to sustain a normal bone metabolism during spaceflight. Especially in early spaceflights, this condition was not always met.
The third confounding influence, and probably the most important one, is the non-standardized level of exercise performed during flights. Exercise, or, more correctly, the strain that muscular pull places on the skeleton, has a beneficiary effect on bone metabolism. In a standing or sitting position under terrestrial conditions, strain is generated by the postural muscles on the so-called weight-bearing bones, as a way of keeping balance in order to stay upright. Thus, muscular pull is generated without being aware of it. But in an upright person under microgravity conditions, there is no muscular activity necessary to prevent the person from falling. Consequently, almost all strain on the weight-bearing bones is absent and this leads to a relatively high bone loss at those sites. The lessened active use of all muscles also leads to a certain degree of bone loss in arms, ribs and vertebrae (Oganov et al., 1992; Davis et al., 1996; Tipton & Hargens, 1996).
These influences can also account for the wide range in percental losses of bone mineral density, as reported in table 3. In spite of the fact that they are presented as average values, they still show a large variability. Individual values are even more variable. Often, multiple BMD measurements are made within one specific anatomical site of a subject. Changes in calcaneal bone density of one particular subject ranged from 30 per cent losses to even increments of up to four per cent (Mack et al., 1967). But the average change in bone mineral mass in one skeletal site is generally a decrease.
An exception to this decrease is the effect on the cranium. The bone mineral density of the cranium is generally found to increase post-flight (Oganov et al., 1992; Schneider et al., 1992; Tipton & Hargens, 1996). This increase might be explained by a better perfusion of the cranial area in microgravity, because an upward directed fluid shift occurs due to microgravity (paragraph 2.3.1). A better perfusion of the cranium also means a better nutritional status, which facilitates bone formation (Arnaud & Morey-Holton, 1990; Duncan & Turner, 1995).
The three confounders mentioned above lead to a large variability in bone losses among sites and individuals. However, this variability cannot be the only cause for some anomalous findings by Oganov et al. (1991b). In addition to changes in the total spine BMD, the separate analysis of the anterior and posterior part of the vertebrae of some crewmembers of the Salyut VII space station showed some remarkable differences (see table 4). Two hypotheses were formulated to explain this finding, namely an extreme site specific effect of exercise, or a protective mechanism of the spinal bone-muscular apparatus, caused by the torsional effects on the vertebral discs (Oganov et al., 1991b).
Table 4: Vertebral bone mineral density changes of Salyut VII crew members. Data are of the members of the first (launch date May 13th 1982; flight duration 211 days) and fourth crew (launch date June 27th 1983; flight duration 150 days). Each crew included two cosmonauts. All changes are presented as percentages as compared to pre-flight bone mineral density values. Data are taken from Oganov et al., 1991b.
|
Anatomical site |
First crew |
Fourth crew |
||
|
anterior vertebrae |
+2.9 |
+4.6 |
+1.8 |
+10.0 |
|
posterior vertebrae |
-3.7 |
-8.1 |
-7.5 |
-11.9 |
|
total spine |
-0.3 |
-6.1 |
-2.3 |
-10.8 |
Theoretically, the upward directed body fluid shift that occurs in microgravity
(see paragraph 2.3.1) leads to a hyperhydration of the vertebral column. This
might increase the rigidity of the intervertebral discs, which during long term
missions gradually would increase and may lead to an interdisc pressure that
is similar to the one in a standing person on earth (Stupakov, 1988; Stupakov
et al., 1990).
Another surprising finding is the reported absence of changes in the mineral
density of the lumbar vertebrae of the second cosmonaut of the third crew attending
the Mir (Grigoriev et al., 1991a). Later that year, it was reported that this
cosmonaut had a five percent increase in BMD after the 366 day flight, but it
was not mentioned whether this figure involved whole body BMD or one particular
site (Grigoriev et al., 1991b). Presumably, the BMD increase in the cranium
and the maintenance of lumbar BMD have played a part in this figure.
The mechanism of bone loss in the spine is perhaps the most complex one because
of itís odd bone structure. The theory put forth by Stupakov (Stupakov, 1988;
Stupakov et al., 1990) might further complicate the already complex theory of
the bone remodelling mechanism during spaceflight. Although BMD changes might
be less severe in the spine than in other weight-bearing bones, the functional
implications of a long term spaceflight might be the same. The durable, elastic
and energy-absorbing features of the spine may still decline, as a result of
structural changes of the bone. The inside architecture of a bone, which dictates
twenty per cent of itís strength (Bosch, 1993), may be detrimented, while itís
BMD remains unchanged or even increases.
2.2.3 - Effects of long-term spaceflight on bone tissue
Although the percentages in table 3 indicate the seriousness of the problem,
BMD losses found so far are not of direct clinical significance (Tilton et al.,
1980). However, the effects of longer duration flights, as might take place
in the future, and the long-term effects of sustained periods of microgravity
combined with the normal ageing process are completely unknown (Grigoriev et
al., 1992). An increased susceptibility to fractures could be expected in people
who engage in such long-term journeys. These possible future problems are not
the only risks that accompany losses in BMD. Bone demineralization also leads
to an increased urine calcium output, which increases the risk of renal calculi
formation. The occurrence of renal calculi is a direct mission-threatening problem,
since present spacecraft do not facilitate in-flight surgery (Lane et al., 1993;
McCuaig, 1994).
2.3 - Other physiological effects of spaceflight
In the remainder of this chapter all other physiological systems that are affected
by spaceflight will be discussed, together with the expected changes of long-term
spaceflights. These physiological systems are the cardiovascular, vestibular,
pulmonary, thermoregulatory, immune, gastro-intestinal, and reproductive systems.
2.3.1 - The cardiovascular system
The microgravity-environment leads to a shift in bodyfluids (Lampe et al., 1992;
Maillet et al., 1994; Newberg, 1994). The reduced gravitational pull causes
a shift towards the central parts of the body, i.e. the thorax cavity and the
head. This shift leads to facial puffiness, often attended with headache and
nausea (Grigoriev et al., 1991b; Maillet et al., 1994; Hargens & Watenpaugh,
1996).
During the first days of spaceflight, blood plasmavolume decreases (Tipton,
1983b; Grigoriev et al., 1991b; Newberg, 1994). This is an effect of the previously
mentioned fluid shift. The exact mechanism of this decrease has not yet been
fully elucidated. As a result from the shift in body fluids, central fluid volume
increases, which leads to several hormonal alterations (i.e. suppression of
aldosterone and vasopressin), which are accompanied by both diuresis (increased
water secretion) and natriuresis (increased sodium excretion; Epstein, 1992).
Thus, the total amount of bodily fluid decreases together with the blood sodium
concentration. This state of dehydration stabilizes after a few days in flight
and is reversed after return to earth.
On the first day in space the stroke volume of the heart is increased compared
to pre-flight values, also because of the body fluid shift. In the days thereafter
stroke volume slowly decreases (Prisk et al., 1993), even under pre-flight values.
A reverse pattern is seen in heart frequency: a strong decrease during the first
day with increasing values thereafter (Prisk et al., 1993; Fritsch-Yelle et
al., 1994). The degree of these increments and decrements are different for
every individual, causing a non-uniform pattern of changes in cardiac output.
The number of red blood cells decrease during spaceflight, as well as the reticulocyte
count and the haemoglobin and erythropoietin concentrations (Grigoriev et al.,
1991b; Tokarev & Andreeva, 1994). The mechanism that causes this so called
"astronaut anaemia" is unclear. A probable cause is the decreased energy demand
under microgravity conditions. In "head down tilt" simulations, the increase
in blood viscosity (caused by the decrease in plasmavolume) is related to a
shorter life span of the red blood cells (Lampe et al., 1992). In microgravity
conditions both systolic and diastolic blood pressures decrease (Crandell et
al., 1994; Goldstein et al., 1995). The effects of long duration spaceflights
do not seem to be any different from the short term effects (Grigoriev et al.,
1991b). After return to earth, the haematological values and blood pressure
return to their pre-flight values.
The initial cardiovascular changes seem to be related to the shift in bodyfluids,
but with a subsequenting individual regulation of cardiovascular parameters.
In flight, the bloodplasma concentration of catecholamines (adrenaline and noradrenaline)
has been reported to drop, which has been used as an indicator for an altered
neural cardiovascular control mechanism (Fritsch-Yelle et al., 1994; Goldstein
et al., 1995). It is likely that a new homeostasis has been formed, which has
been shown to last for at least a 366 day-stay (Grigoriev et al., 1994), which
in itself is not harmful. Flights of longer duration are not likely to disturb
this new homeostasis. After return to earth all cardiovascular values return
to their pre-flight values (Prisk et al., 1993).
2.3.2 - Vestibular and receptor functioning
The absence of normal gravitational forces imposes unusual stimuli on the vestibular
organ. A common problem during the first few days in space, and sometimes also
after return to earth, is "space motion sickness" (SMS), which is accompanied
by the same symptoms as motion sicknesses during car or boat rides, such as
dizziness, nausea and vomiting. These symptoms disappear after a few days (Newberg,
1994). The most commonly accepted theory for this phenomenon is the unusual
mismatch between visual and vestibular stimuli. This mismatch causes nausea
and related symptoms.
After spaceflight some persons experience extreme dizziness or even periods
of syncope (the impairment of consciousness associated with a loss of postural
tone; Schraeder et al., 1994). This phenomenon is known as "orthostatic intolerance"
(Fritsch-Yelle et al., 1994; Hargens, 1994; Convertino, 1996). These problems
of the orthostasis are related to the cardiovascular changes as described in
the previous paragraph. The lowered blood pressure during flight is sometimes
too low to push the blood to the level of the brain against the suddenly re-occurring
gravitational forces. With the return of blood pressure to terrestrial values,
the problems of syncope disappear.
Vestibular changes after long term flights might indicate a change in receptor
functioning. In two Russian cosmonauts of the third crew attending the Mir space
station, the oscillation range of the centre of gravity while standing upright
was increased by 50% and 11% respectively. Furthermore, decrements in the threshold
of posture correction responses to perturbations, prolongation of posture recovery
and increased electromyographic cost of standing were reported (Grigoriev et
al., 1991b). These and other motor system parameters did not return to pre-flight
values until fourteen days after return. Similar findings have been reported
in cosmonauts after a stay in the Salyut VI or Salyut VII space stations (Vorobyov
et al., 1983; Kozlovskaya et al., 1990).
These findings might indicate irreversible changes in balance keeping ability.
The cause of these changes is unknown, but might be related to either a loss
in muscle strength or to damaged vestibular receptors due to cosmic radiation.
If the first is the major cause, the vestibular changes should diminish with
the same degree and in the same time course as the changes in the postural muscles
are reversed. But the long term effects of cosmic radiation are still unknown
and could be irreversible (Newberg, 1994).
2.3.3 - Pulmonary functioning
In humans, lungvolume decreases under microgravity conditions. Due to the changes
in forces applied on the torso, the thorax decreases in size (Engel, 1991).
Total lung capacity is also decreased, largely because of a decrease in residual
volume and to a lesser extent because of a decrease in expiratory reserve volume
(Engel, 1991; Elliot et al., 1994). These decrements do not restrain crew members
in any activity during spaceflight (Engel, 1991). Since these parameters return
to their normal values immediately after return to earth, it is believed that
they are a direct result from microgravity, and that they are of non-adaptive
nature (Prisk et al., 1993; Elliot et al., 1994). Therefore, no complications
are expected during long-term spaceflights.
2.3.4 - Temperature regulation
In a head down tilt simulation of weightlessness the sweat response has been
shown to be deteriorated. Consequently, core temperature increases more rapidly
during exercise and it takes a longer period to recover from such a rise (Crandell
et al., 1994). These effects are similar to responses during spaceflight (Pandolf
et al., 1995) and can be attributed to a disturbance in skin blood flow. This
disturbance is caused by a decrease in plasma volume (Crandell et al., 1994).
This dehydration seriously affects the thermoregulatory control mechanism, which
can lead to dangerously high core temperatures. In-flight, no thermoregulatory
problems have been reported, but computer simulations have shown that during
re-entry into the earth atmosphere, rectal temperature can rise above 39įC,
despite the ventilated cooling garments worn at that time (Pandolf et al., 1995).
This problem has been recognized since the early days of spacetravelling and
has been counteracted by isotonic water supplementation just hours preceding
re-entry (Grigoriev et al., 1991b; Fritsch-Yelle et al., 1994; Grigoriev et
al., 1994). This countermeasure is still executed with satisfactory results,
although computersimulations and experimental evidence suggest that a hypertonic
solution has even better results (Srinivasan et al., 1992). As previously mentioned,
the state of dehydration in space is actually a new homeostasis. Therefore,
the degree of dehydration is not likely to be worse during long-term missions.
Pre-landing water supplementation, either isotonic or hypertonic, seems to remain
the proper countermeasure against anticipated problems during re-entry and shortly
thereafter.
2.3.5 - The immune system
After a one year stay in the space station Mir, two cosmonauts showed elevations
in the levels of several indicators of an immune response, such as blood immunoglobulins
and lymphocyte production of alpha- and gamma-interferons (Grigoriev et al.,
1991b). The increase in secretion of the cytokines interleukine-3 and interleukine-6
found in animal experiments also indicates an increased activity of the immune
system (Hon et al., 1994; Miller et al., 1995). It has also been shown that
spaceflight induces significant changes in interleukine-6 secretion in humans
immediately after launch (Stein & Schluter, 1994).
The observation that the immune system is activated during spaceflight is not
surprising. Considering the stresses placed upon the human body (i.e. dehydration
and atrophy of both muscle and bone), the body would be expected to be in a
state of alert. A more interesting question, is whether the immune system itself
is affected during spaceflight, leading to a deteriorated defence mechanism.
This deterioration is likely to take place, since reduced reactivity of several
immunological parameters has been found (Konstantinova et al., 1992; Taylor,
1993; Stein & Gaprindashvili, 1994). Whether this is caused by the mere
absence of gravitational forces, the stress of living in a confined and unusual
place, alterations in cell structure due to ionizing radiation, or to a combination
of these three factors remains to be determined. Since the most extensive changes
have been present after prolonged flights (Konstantinova et al., 1993), it is
important to carefully monitor all immunity disturbances during future flights
of even longer duration.
2.3.6 - The gastro-intestinal tract and the reproduction organs
The effects of spaceflight on the gastro-intestinal tract and the reproduction
organs are largely unknown. In most flights, which are shorter than seven days,
no serious problems have been detected. Anecdotal information suggests that
constipation is common in flight, but no prevalence data are available (Lane
et al., 1993). However, constipation could just as easily be attributed to problems
in personal hygiene associated with a weightless environment, as to any physiological
malfunctioning such as a reduced bloodflow in the splanchnic region or changed
gastro-intestinal functioning.
Human reproduction could be affected by either the microgravity environment
or by sustained exposure to radiation. The prevalence of pregnancy in space
is not yet an issue of direct importance, but when missions will last several
years it could very well become one. The state of microgravity may have impact
on in utero embryonic development and reproductive physiology in both males
and females, as evidenced by animal studies (Jennings & Santy, 1990). Prolonged
exposures to ionizing radiation could have serious effects on the human reproduction
organs, such as infertility or genetic disorders. These possible effects will
remain speculative, however, until longitudinal research is performed in this
area (Jennings & Santy, 1990).
2.4 - Concluding remarks
One might start to wonder whether it is all worth it to send people into an
environment which has such a destructive effect on so many physiological functions,
even aside from the huge amount of monetary funds that are related to space-exploration.
However, it is beyond the scope of this paper to take up this discussion and
it is beyond the authorís ability to give a satisfactory answer to this multi-angled
moral question. From a medical point of view, this question does not necessarily
have to be answered. The fact that people are sent into space for any reason
makes it morally obligatory to investigate the effects of that environment on
the human body.
However, the position of the medical researcher is not completely passive. The
area of physiology can benefit from the unique research ability the weightless
environment administers. Human responses to a stay in space show remarkable
resemblances to the physiological changes that occur with ageing (Ray, 1991;
Roy et al., 1991; Bloomfield, 1997). Thus, a study of the effects of spaceflight
gives an opportunity to study the human ageing process. Also, studying biological
processes in a weightless environment presents a clear view of the actual processes
occurring inside biological systems. Without the confounding presence of gravity,
biological systems can be understood better (Nicogossian, 1994). One of the
goals of NASA is to improve the quality of life on earth through space-related
research (Sulzman, 1991; Taylor, 1994).
A lot of effort is put in the amelioration of all disadvantageous effects of
spaceflight discussed above. Current optimal medication, including the performance
of exercise, and nutrition do not nullify these problems, but do make human
life under microgravity conditions possible for as long as 437 days (Bachl et
al., 1996). The planned flights of longer duration (i.e. several years) impose
new challenges on the currently used in-flight programs.
The principal preventive countermeasure used in spaceflight is exercise (Grigoriev
et al., 1992; Davis et al., 1996). Specific regimens of exercise are thought
to be beneficiary to muscles, bone, cardiovascular functioning and even to the
immune system. With an increased muscle strength, the performance in balance
keeping tasks might also improve and with a better functioning of the cardiovascular
system, both the thermoregulatory problems during re-entry and the problems
of orthostatic intolerance just after re-entry might be diminished, although
the last aspect remains controversial (see e.g. Klein et al., 1977; Tallarida
et al., 1991; Siconolfi et al., 1994; Shvartz, 1996). The effects of prolonged
exposure to cosmic radiation remains to be a problem with unknown consequences
that is not possible to solve with some kind of exercise.