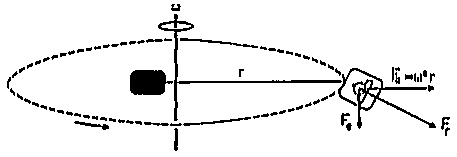
Figure 2.1. Diagram showing the supine position of the subject in the gondola of the centrifuge. Because the gondola is free-swinging it aligns itself with the resultant force Fr which is the vector sum of gravity Fg and centrifugal force Fc.
| Intro | Chap. 1 | Chap. 2 | Chap. 3 | Chap. 4 | Chap. 5 |
| Summary | Concl. Remarks | Bibliography | Samenvatting | CV | Publications |
Chapter 2
Vestibular adaptation to sustained hypergravity
Introduction
The aim of this experiment was to identify vestibular adaptation to hypergravity by comparing ocular responses to body tilt and rotation, before and after an one-hour +3Gx centrifuge run. Because the otolith organs are being stimulated by gravito-inertial force, we expected to find changes in otolith-induced eye movements due to adaptation to the new 3G level. For this purpose we measured the ocular torsion (OT) response to static lateral body tilt (roll). This response, also indicated as ocular counterrolling, is considered to reflect the function of the otoliths (e.g. Colenbrander 1963; Miller 1962; Miller and Graybiel 1971). Hence it would suffice to examine otolith adaptation in static conditions only. However, previous experiments with prolonged centrifugation have shown that subjects experienced the strongest after-effects during head movements, in particular when these head movements were about an off-vertical axis (Bles et al. 1989; Bles and De Graaf 1993; De Graaf and De Roo 1996). This suggests a disturbed otolith-canal interaction. Therefore we also registered the dynamic OT response (or torsional VOR) during sinusoidal body roll, stimulating the otoliths and the semicircular canals simultaneously. To distinguish the contribution of the otolith organs from that of the semicircular canals, we measured the response to the same roll stimulus in two conditions, once about an earth-horizontal axis (body upright), and once about an earth-vertical axis (body supine). Thus the two conditions differed with respect to stimulation of the otoliths. In the upright condition, the otolith input from gravity was modulated because of the continuously changing head tilt. Conversely, in the supine condition the otolith input remained constant since the rotation was orthogonal to gravity.
In addition to these OT measurements we studied two more variables. First, in another static tilt condition we asked the subjects to align a visual line with the apparent vertical. This was done to relate possible otolith adaptation to alterations in perception of the body orientation with respect to gravity. Second, we examined the horizontal VOR during earth-vertical rotation (yaw) to make sure that we did not overlook adaptation in the semicircular canals. We had the notion that such could be the case, since subjects previously reported illusory surround motion during head movements about all three major axes, including the yaw axis.
Our hypothesis was that the centrifuge run would primarily affect otolith-induced responses, ie. in conditions of static body tilt and body roll about an earth-horizontal axis. More specifically, we expected that the sensitivity (gain) of the otoliths would be reduced due to adaptation to the sustained high G-load. Nevertheless, we did not rule out to find changes in canal-induced eye movements.
Methods
Centrifuge
The centrifuge was located at the Netherlands Aerospace Medical Centre (NLRGC), next to the TNO Human Factors Research Institute where the measurements took place. It had a free swinging gondola suspended to a 4 m long arm. For the purpose of these experiments, the aircraft seat inside the gondola was removed and replaced by a mattress, supporting the subject in supine position. This was done to prevent blood pooling in the legs during the long duration centrifuge run. The body was inclined forward over about 10º due to the dimensions of the gondola. The centrifuge accelerated at 0.1 G/s2 up to a resultant G-force vector of 3G. Since the subject was supine and the free-swinging gondola remained collinear with the resultant force, the stimulation was directed along the subject’s x-axis (Figure 2.1). The subjects was asked to avoid head movements during the run.
All subjects gave their informed consent before the experiment. Each subject underwent a medical checkup. Details on medical monitoring during and after the run were reported elsewhere (Bles et al. 1989). There was continuous verbal contact possible between subject and operator, who could also observe the subject on a video screen. At any time the subject had the possibility to end the centrifuge run by himself, or by asking the operator. Transportation between the TNO and the NLRGC was done by car, although the subjects had to walk about 50 m inside each institute, which included two staircases. In total 18 healthy subjects participated in this experiment. Maximally two subjects were tested each day. The first subject started between 9:00 and 9:30 am at the TNO institute, where OT was measured in the tilt chair. These measurements took about one hour, so that the centrifuge run could start around 11:00 am. During this run the tilt chair session with the second subject was carried out, so that this subject’s centrifuge run could start shortly after completion of the first.

Figure 2.1. Diagram showing
the supine position of the subject in the gondola of the centrifuge. Because
the gondola is free-swinging it aligns itself with the resultant force Fr
which is the vector sum of gravity Fg and centrifugal force Fc.
OT during static and dynamic tilt
OT was measured during passive static and dynamic body tilt about the naso-occipital axis (roll, or x-axis). Subjects were seated in the TNO tilt chair, the rotation axis being centered between both eyes. The subject’s head was supported by a head rest, and the body was firmly strapped to the chair. Ocular torsion measurements were performed once before ("pre-test") and once after ("post-test") the centrifuge run. The post-test generally took place 20 min. after the centrifuge run.
In the static tilt condition, subjects were seated upright and tilted to successive angles of 10, 20, 30, 42, and 57º to the right (clockwise). OT was recorded on videotape (see next section) after 20s of constant tilt, when the influence of the semicircular canals was considered to have subsided. A video image of each eye taken in the erect subject served as reference for the quantification of OT in tilted positions. In the dynamic condition, OT was registered during ten cycles of passive sinusoidal body roll at the frequency of 0.25 Hz and an amplitude of 45º. First, this stimulus was applied in an upright body orientation (roll axis parallel to the earth horizontal), and after a 5 min. rest, in a supine body orientation (roll axis parallel to the earth vertical). In the upright condition, oscillation was symmetrical about the vertical (0º offset).
Video-oculography (VOG)
Eye movements of both eyes were recorded simultaneously on video tape by means of two small CCD video cameras mounted to a head set and placed 2 cm in front of the eyes, the optical axes approximately aligned with the visual axes. Except for a point source of light, fixed to each camera for illumination of the eyes, the measurements were performed in darkness under a hood. Subjects were asked to look straight into the cameras, using a reflection dot on the camera lens for fixation, so that horizontal and vertical eye movements were negligible. There were no visual cues for orientation to vertical. The subjects were encouraged to keep their eyes open and refrain from eye blinks during the actual eye movement recordings.
For analysis of single shot images in the static condition, the semi-automatic method described by Bos and De Graaf (1994) was used. The time series recordings in the dynamic paradigm were analyzed with a sample frequency of 50 Hz, using the automatic method that we developed especially for this purpose (Groen et al. 1996), as described in Chapter 1 of this thesis. For the analysis of the dynamic OT response the first three cycles were disregarded to allow for build up of the response. From the remaining seven periods two parameters were calculated. First, the torsional VOR gain was calculated by dividing the amplitude of the slow component velocity (SCV) by the amplitude of the stimulus velocity (Vmax = 70º/s). The SCV amplitude was obtained by a sinusoidal fit on the OT signal after digital differentiation and interactive saccade removal (see Figure 2.4). Second, the mean amplitude of the original OT signal was calculated to quantify the total excursion range of torsional eye position, which we will refer to as OT-range. Whereas the SCV gain is related to the dynamics of the slow component only, the OT-range also concerns the influence of saccades.
Subjective vertical during static body tilt
In nine subjects we measured the subjective vertical during static tilt in clockwise direction. In this case, the camera in front of the right eye was replaced by a miniature computer monitor. On this monitor a red line was presented at a virtual distance of 60 cm in front of the subject’s eye. The length of the line subtended a visual angle of about 10º. The line could be rotated by means of a tracker ball mounted on the armrest of the tilt chair. At each angle of static body tilt the subject was asked to set the line parallel to the apparent vertical. The initial position of the line was always upright in the coordinates of the monitor, which was head-fixed and thus aligned with the subject’s z-axis. The angle between this null position and the final setting by the subject will be referred to as the "subjective vertical". In this way, the subjective vertical gives a direct estimate of the perceived body tilt. Because visual judgments of the vertical are confounded by OT (Howard 1982; De Graaf et al. 1992), we corrected for the amount of torsion that was measured in the right eye during the static OT trial.
Horizontal VOR during yaw rotation
Finally, we measured the horizontal eye movements in response to a velocity step rotation about the vertical yaw in nine subjects. Horizontal eye movements were recorded using Electro-oculography (EOG), as described by Bos and De Graaf (1997). The stimulus profile consisted of an acceleration of 90º/s2 up to a constant velocity of 90º/s ("velocity step"), which was maintained for 1 min. followed by a deceleration to a stop. This profile was done for leftward and rightward rotation, respectively, with a few minutes rest in between. The gain and dominant time constant for the per-rotatory and post-rotatory phase were estimated by fitting a single exponential function to the velocity signal after saccades had been removed by means of a median filter with a time window of 500 ms.
|
Score |
(Dis)comfort |
|
0 |
No problems |
|
1 |
Dizzy |
|
2 |
Stomach awareness |
|
3 |
Nausea |
|
4 |
Very nauseated (edge) |
|
5 |
Vomiting |
Table 2.1. Miscery scale (MISC)
Motion sickness symptoms (SIC)
From previous centrifuge experiments it is known that about 40-50% of all subjects develop symptoms of motion sickness after a prolonged exposure to 3G. This phenomenon was designated Sickness Induced by Centrifugation (SIC: Bles et al. 1989; Ockels et al. 1990; De Graaf and De Roo 1996). Since in the present study we looked for the vestibular mechanism underlying the aftereffects of centrifugation, including SIC, we asked the subjects to report their subjective (dis)comfort at regular time intervals, before and after the centrifuge run. For this we used a six-point scale (MIscery SCale, MISC), as shown in Table 1 (De Graaf et al. 1992; Wertheim et al. 1992). A subject was considered to suffer from SIC, when their maximum score was 3, 4, or 5.
Results
All 18 subjects who underwent the centrifuge run mentioned visual motion illusions during head movements (oscillopsia) afterwards, similar to earlier reports (Bles and De Graaf 1993). Six subjects developed serious symptoms of SIC after the run, lasting between 2-6 hours. Due to technical problems with the tilt chair we were not able to perform vestibular testing in two subjects after the centrifuge run. Moreover, the dynamic OT could not be determined accurately in an additional five subjects, because of various artifacts, such as "droopy" eye lids or indistinct iris structures (dark brown eyes). The data of these subjects was excluded from the analyses, so that the torsional VOR of 11 subjects will be presented. This group included four of the subjects who suffered from SIC.
OT response during static tilt
The amplitude of OT showed large intersubject variability, which is common for this response. Effects were tested using an ANOVA within subjects design (factors: pre-post; left-right eye; tilt angle). There was a main effect for tilt angle (F=61.33; df=4,40; p<0.001), and a main effect for pre/post-test (F=7.88; df=1,10; p<0.05). The mean OT curves from the pre-test and the post-test are shown in Figure 2.2 (pooled for both eyes, since the response was the same for both eyes). On average, OT was 10% smaller in the post-test with respect to the pre-test.
Subjective vertical during static tilt
Figure 2.3 shows the mean setting of the visual line of nine subjects as function of static clockwise body tilt. On the whole, subjects underestimated the angle of body tilt (A-effect). More importantly, the performance did not change as a result of
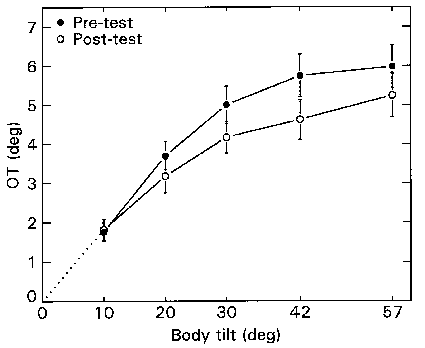
Figure 2.2. Mean OT
response as a function of clockwise static body roll, before and after the one
hour centrifuge run. Positive OT values denote anti-clockwise rotation (ie.
compensatory to the tilt). The OT The response was about 10% smaller in the
post-test than in the pre-test. The bars indicate standard errors of the mean
(n=11).
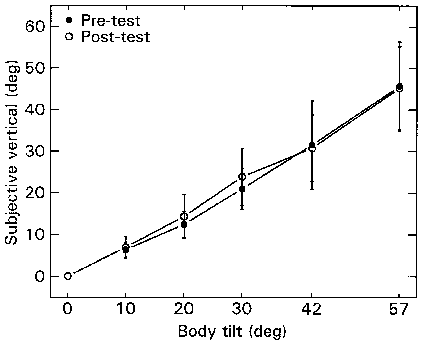
Figure
2.3. The mean subjective vertical as indicated by the setting of a line
to the apparent vertical, before and after centrifugation. The bars are the
S.E.M.(n=9). Line settings under the dotted line denote underestimation of tilt
(A-effect).
centrifugation. Correction of the line settings with the corresponding OT data did not change this result. The only main effect was for body tilt (F=105.12; df=4.32; p<0.001).
OT response during dynamic tilt
The OT response to sinusoidal body roll in both body orientations is shown in Figure 2.4 for one subject. The recordings show a compensatory "slow component", which appears to be interrupted by saccades more frequently in the supine than in the upright orientation. This aspect will be discussed in more detail in Chapter 3 of this thesis.
For all calculations, data from both eyes have been averaged. The group’s mean values and standard errors of the mean are listed in Table 2.2. The pre-test SCV gain was smaller in the upright condition than in the supine condition (Student’s t-test, p<0.05). The mean gain was 0.31 in upright and 0.35 in supine. On the other hand, the mean OT-range in upright was twice as large as in supine, due to a difference in saccadic activity. In both conditions there was a mean phase lead of about 40º of the response relative to the inverted stimulus (to account for the fact that an ideal compensatory response would be in anti-phase with the stimulus).
|
Gain tVOR |
OT-range (º) |
Gain hVOR |
Tc hVOR (s) |
|||||||
|
Upright |
Supine |
Upright |
Supine |
Per-rot |
Post-rot |
Per-rot |
Post-rot |
|||
|
Pre-test |
0.31 ±0.01 |
0.35 ±0.01 |
7.8 ±0.5 |
3.9 ±0.5 |
0.59 ±0.20 |
0.61 ±0.18 |
15.7 ±1.7 |
18.7 ±2.2 |
||
|
Post-test |
0.34 ±0.02 |
0.35 ±0.02 |
8.2 ±0.7 |
3.0 ±1.8 |
0.60 ±0.15 |
0.63 ±0.15 |
12.8 ±1.1 |
15.2 ±1.6 |
||
Table 2.1. Means and S.E.M. of the gain and OT-range of the OT response (tVOR) to sinusoidal body roll, and the gain and time constant (Tc) calculated from the horizontal VOR (hVOR) to yaw rotation.
Neither the OT-range nor the phase of the response were different between pre-test and post-test. In the upright condition, all but one subjects showed a larger response in the post-test than in the pre-test. The mean SCV gain increased from 0.31 to 0.34 (Figure 2.5a). In the supine condition, the averaged gain remained the same (0.35). The individual responses, however, showed divergent behavior: the gain increased in six subjects, remained constant in one subject, and decreased in four other subjects. As a result, the apparent interaction in Figure 2.5a between upright/supine and pre-test/post-test was not statistically significant (p=0.18). It is interesting to note that the four subjects who showed a reduced gain, were also the ones who suffered from SIC. In Figure 2.5b the same data are plotted again, but now differentiated between those subjects with SIC symptoms and those without. It can be seen that the gain increase was approximately parallel in both conditions, except for the four SIC subjects.
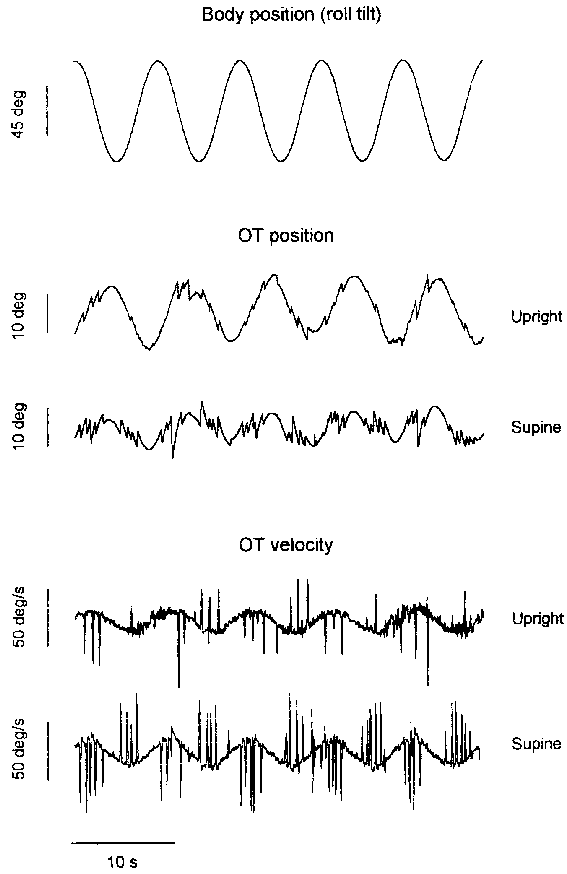
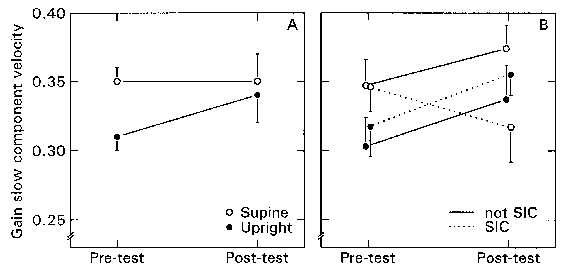
Figure 2.5. a. Gain
of slow component velocity of torsional VOR. Mean values of 11 subjects in pre-test
and post-test for upright and supine body orientation (with contribution and
without contribution of the otoliths, respectively). The bars denote the S.E.M.
In the pre-test the upright gain was higher than the supine gain. The upright
gain was significantly increased in the post-test. The mean supine gain, however,
remained constant. b. The same torsional VOR data as in Fig. 2.5, but
now distinguished between the four SIC subjects (dotted lines) and seven non-SIC
subjects (solid lines). It appears that the SIC and non-SIC subjects showed
the same gain increase in the upright condition, but that the mean gain of the
SIC subjects dropped in the supine condition after centrifugation, whereas the
mean gain of non-SIC subjects showed a slight increase. Bars are the S.E.M.
Horizontal VOR during rotation about yaw-axis
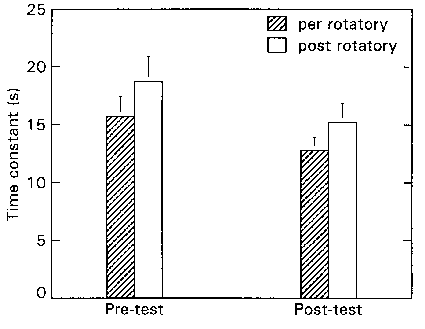
The mean gain and mean time constant of the per- and post-rotatory phase of the horizontal VOR measured in nine subjects are listed in Table 1. The mean gain was about 0.60 in the per- and post-rotatory phase, and was the same before and after the centrifuge run. The dominant time constant (Figure 2.6), on the other hand, was on average 3 s longer in the pre-test than in the post-test (F=29.21; df 1,14; p<0.001). Also, the time constant observed in the post-rotatory phase was generally longer than that observed in the per-rotatory phase (p<0.01). No differences were found between subjects with SIC and subjects without.
Discussion
In this study we examined changes in vestibular parameters due to sustained exposure to hypergravity in human subjects. In this way, we hoped to substantiate the effects of postural instability and motion sickness which have been observed after one hour centrifugation at 3G (Bles et al. 1989; Ockels et al. 1990; Bles and De Graaf 1993; Albery 1994; Bles et al. 1995; De Graaf and De Roo 1996). Because we expected that a high G-load would especially influence the otolith function, we measured the OT response to static body tilt. Previous observations pointed to alterations in the canal-otolith interaction, which is why we included the OT response to dynamic body tilt. To verify that no direct changes in the functioning of the semicircular canals occurred, we also investigated canal-induced horizontal eye movements. The major finding was that the gain of the static OT response was consistently reduced by 10% after the centrifuge run of one hour. The results from the dynamic OT measurements were less explicit. In both body orientations the gain of the compensatory slow component was higher in the post-test by about 10% in all subjects, except in the four subjects who suffered from SIC. These subjects showed the same trend in the upright condition, but their response was reduced in the supine condition. Finally, the gain of the horizontal VOR remained constant after centrifugation, but the time constant was on average 3 s shorter in the post-test compared to the pre-test.
Unfortunately we had to disregard the OT data from five of the eighteen subjects, mainly because the automatic pattern recognition algorithm to determine OT was still in development at the time of the data collection, and we did not know the exact criteria for accurate analysis. All data were collected using a visible light source, while dark brown pigmented eyes should preferably be illuminated by infrared light, so that iris structures become more distinct.
Static OT
The OT response to static tilt suggests that the otolith function adapted to a higher G-load by a gain reduction. It seems reasonable that otolith adaptation would occur, since this sensory system must detect small linear accelerations against relatively large background stimulation by gravity. Normally, the otolith system operates in an invariant force field of 1G, with an optimally adjusted basic activity ("set-point") and sensitivity. Changing the background level of 1G will shift the set-point away from its ideal position. Adaptation will restore the operational range of the system within its normal limits. Thus, in a 3G environment the otoliths would shift their set-point to a higher value. Assuming that re-adaptation to normogravity is not immediately, the higher setting is likely to result in a smaller response after centrifugation. The direction of the OT change (a decrease in amplitude) in this experiment is in agreement with this.
From this reasoning we would expect that the static OT response would increase after exposure to 0G (weightlessness). Previous studies on vestibular adaptation to spaceflight, however, suggest the opposite (Vogel and Kass 1985; Arrot and Young 1986; Hofstetter-Degen et al. 1993; Dai et al. 1994). This discrepancy may be explained by the unique property of weightlessness that there is no effective gravitational force to support spatial orientation. On earth, otolith-induced OT is considered an orientational response which tends to maintain the eye position with respect to gravity. In space, the otolith organs no longer register gravity, but only receive stimulation from linear accelerations induced by head movements. It has been postulated that in weightlessness all otolith signals become interpreted in terms of head translation. This is known as the "tilt-translation reinterpretation theory" (Parker et al. 1985). Accordingly, the OT response to head tilt is strongly reduced or absent in spaceflight (Hofstetter-Degen et al. 1993; Clarke et al. 1993). Moreover, linear motion along the subject’s y-axis, which normally induces OT, primarily produces horizontal eye movements after spaceflight (Arrot and Young 1986). Hence, the effects of weightlessness on the OT response presumably result from sensory-rearrangement which is related to the reduced demand for orientational responses. The reduction in OT gain observed in the present study may be based on a different mechanism, because in hypergravity there is a gravitational force to allow for orientational responses. The fact that we measured an effect during tilt about the subject’s x-axis, whereas the resultant G-load was directed along the x-axis, implies that a central mechanism is involved.
The effect on the OT response was not mirrored in the settings of a visual line to vertical. Theoretically, a reduction in the sensitivity of the otolith system could have consequences for the perceived angle of body tilt, and thus for the judgment of the verticality of a line. According to the following reasoning, the perceptual effect could even be larger than the oculomotor effect. Imagine that the subjective vertical would depend entirely on the information provided by the otolith system. Imagine also that a certain OT angle would strictly correspond to a certain angle of body tilt specified by the otolith system. Then the expected change in the perceived angle of body tilt could be obtained by looking at the post-test value of static OT at a certain angle of body tilt, and finding the angle of body tilt which produced the same amount of OT in the pre-test (by drawing a horizontal line through an OT value in the post-test curve in Figure 2.2, and determining at which angle of tilt the line crosses the pre-test curve). Nevertheless, there was no connection between the two measures, as can be seen by comparing Figure 2.2 and 2.3. Other sensory senses, such as non-vestibular proprioceptors (De Graaf et al. 1992), may have compensated for the reduced otolith gain in the determination of the subjective vertical. Previous centrifuge experiments have established an effect on the control of postural balance, so that we can not eliminate the possibility that reduction of the otolith gain (even if this is only 10%) has larger impact in situations that require more active behavior than does passive static body tilt. From this perspective dynamic SV measurements would have been useful.
Torsional and horizontal VOR
The profile of the centrifuge run consisted of a 30s acceleration up to a constant velocity of about 150º/s, which was sustained for one hour before the centrifuge was decelerated to a stop in 30s. Thus there were only two brief periods of angular acceleration, and we did not expect main changes in the functioning of the semicircular canals due to the centrifuge run. We did find a 10% increase of the slow component gain of the torsional VOR. In all but the four SIC subjects this trend was apparent in both body orientations, which excludes an otolithic origin and argues for a change in the canal-induced VOR. The latter was not confirmed by a change in the gain of the horizontal VOR. Spaceflight studies have provided inconclusive evidence on the effects of an altered G-environment on the canal-induced VOR. In the Spacelab-1 mission, Benson and Vieville (1986) did not observe changes in the horizontal VOR gain in human subjects (during active and passive oscillation). In monkeys, neither the horizontal VOR gain (Cohen et al. 1992), nor the vertical VOR gain (Dai et al. 1994) was found to be affected by weightlessness. On the other hand, Berthoz et al. (1986) measured an increase of the vertical VOR in humans on the first day after spaceflight. Clarke et al. (1993) measured a reduction in the active torsional VOR in human subjects during the first four days inflight, although Cheung et al. (1992) did not find any differences in the torsional VOR during parabolic flight. One factor contributing to these variable results may be the restricted number of subjects that is typical for studies in space. Another explanation is that possible adaptation processes in the sensory system may be concealed by the high plasticity of the VOR, as was suggested by Dai et al. (1994). They did not find changes in the horizontal and vertical VOR gain in monkeys after a two week spaceflight, while the activity of the primary afferents of the semicircular canals was found to be increased in the same animal in an accompanying study (Correia et al. 1994).
It is unlikely that the different post-test gain of the torsional VOR observed in the SIC subjects in the present study, reflects a causal factor in the etiology of SIC. First, the stimulus itself was not experienced as provocative. Second, the response of the SIC subjects only differed from the other subjects in the supine roll condition, in which the rotation was about an earth-vertical axis, whereas motion sickness symptoms were induced by head movements about an off-vertical axis. For that reason, a difference in the upright roll condition would have been more compatible with the subjective reports. It is possible that the torsional VOR gain in the SIC subjects was related to their state of alertness. Even though subjects were encouraged to stay alert in all conditions, the measurement in a supine body position in the dark may have invoked drowsiness especially in these subjects. In contrast, the upright position may have provided sufficient arousal for a normal response.
Surprisingly, the SCV gain of the torsional VOR in the pre-test appeared higher in the upright condition than in the supine condition, suggesting that the otolith function counteracted the canal-induced response. In view of the low gain of the torsional VOR (the highest mean gain that we found was about 0.37) we had expected that the combination of canal and otolith stimulation would yield a larger response than canal stimulation alone. In another experiment we investigated this response in more detail. The results of this experiment are described in Chapter 3 of this thesis. For now we will conclude that the otolith inputs have small and variable effects on the magnitude of the slow component of the torsional VOR. More important effects include modulation of the phase of the response at frequencies below 0.2 Hz, and modulation of the OT range. Since both parameters were unchanged after centrifugation, these otolith components provided no evidence for otolith adaptation.
Finally, the decrease in the time constant of the horizontal VOR is interesting with regard to a possible canal-otolith interaction. In general, the falling time constant of the VOR for rotations about an earth-vertical yaw axis is longer than the time constant of the signals from the semicircular canals, due to a brainstem circuitry designated "velocity storage" mechanism (Raphan et al. 1979). The time constant of velocity storage is affected by inputs from the otoliths. It can be considerably reduced when the head is suddenly tilted ("tilt dumping" ), and it is shorter during off-vertical axis rotations (Dai et al. 1991; Tweed et al. 1994b). It is tempting to attribute the reduced time constant in the post-test to altered otolith inputs that feed into the velocity storage mechanism. The static OT data may confirm this. An interesting hypothesis is that, after the centrifuge run, the velocity storage is inaccurately informed about the orientation of the rotation axis, and erroneously "assumes" that the rotation is not about a vertical axis, resulting in a shorter time constant. This can be visualized as if the system is incorrectly spatially oriented because it is aligned with the resultant vector sum of actual gravity and some "residual" +3Gx vector from the centrifuge run (like an inverted G-excess phenomenon), until re-adaptation is complete. Psychophysical evidence for this is provided by the sensation reported by most subjects, that they feel inclined forward in the gondola after deceleration from a prolonged centrifuge run (Bles and De Graaf 1993). This sensation normalizes only after a couple of minutes, although even after 30 minutes a few subjects showed a measurable bias of backward tilt when they were asked to align their body with vertical (Bles et al. 1989). Moreover, Dai et al. (1994) demonstrated in a spaceflight study that the spatial orientation of the velocity storage mechanism is indeed capable to adjust to the G-environment. At any rate, the shortened time constant is a strong indication that the centrifuge run had consequences for the canal-otolith interaction.
Model for SIC
So far the only difference found between subjects who suffered from SIC and those who did not, concerned the response to supine body roll. As explained above, we do not see how this can play a role in the etiology of SIC. Instead, it is likely that this difference was a consequence of their malaise, and not a cause. All other parameters examined in the present study do not differentiate between SIC and non-SIC subjects, and therefore have no predictive value for the susceptibility for SIC. Although the changes in ocular responses that we measured were relatively small, they may explain the illusory surround motion during head movements. On the other hand, the difference in magnitude of the effects that were subjectively reported and the effects that were objectively registered may be due to different time scales by which perceptual changes take place as compared to sensorimotor changes. Indeed, it has been shown in human subjects living in an enclosed rotating room, that perceptual and oculomotor reactions did not follow the same course (Guedry 1991).
Based on the observation that after centrifugation visual motion illusions may be induced by all head movements (including yaw-motion), but that motion sickness symptoms are only provoked when the orientation of the head is changed relative to gravity (roll and pitch), it was proposed that SIC is generated by discrepancies in the internal representation of the vertical (the subjective vertical; Bles and De Graaf 1993; Bles et al. 1997). This probably is a more accurate interpretation than the commonly accepted "conflict theory" on motion sickness, which states that motion sickness arises when the information from different sensory systems is "at variance with one another or with what is expected from previous experience" (Reason and Brand 1975).
The new model assumes that motion sickness only arises when there is a conflict about the direction or magnitude of the subjective vertical. The subjective vertical is determined based on the integrated information from the eyes, the vestibular system and the non-vestibular proprioceptors. Normally the information from these systems is congruent. However, when one or more of these systems adapt to earth-anomalous gravity, this expectedly influences the computation of the subjective vertical. It should be noted that an inaccurate subjective vertical does not implicitly produce motion sickness. For instance, the present study demonstrated that passive body roll in the dark was not experienced as provocative, whereas active roll motion of the head in the light was. Furthermore, static situations were not provocative at all. Apparently some self-generated action is needed to perceive the discrepancy between the subjective vertical and the reafferent feedback obtained by this action (De Graaf et al. in press). But even then it is still unclear why some subjects suffer from SIC while others don’t. A possible explanation is that, despite comparable physiological changes, subjects may assign different relative weightings to the information provided by the various sensory systems, leading to perceptual differences.
An alternative theory on the generation of space sickness is based on the assumption that there are minor anatomical and physiological differences between the left and right utricle (Baumgarten and Thümler 1978). On earth, these differences would be fully compensated for, but under hypo- and hypergravity these differences would become pronounced, requiring new adjustments. During this process motion sickness may result. The asymmetry measured in OT during parabolic flights may be a manifestation of this utricular imbalance (Markham and Diamond 1991). We did not find (a change in) the asymmetry between the left and right eye. Moreover, the OT response to static body tilt was similar in SIC and non-SIC subjects, so that we did not verify an effect of hypergravity on utricular imbalance, as a possible cause in the generation of SIC.
Conclusions
The results from this study suggest that the sensitivity of the otolith function was reduced by 10% after a long duration +3Gx centrifuge run. This was observed by a decrease in static OT amplitude. Although the effect was not present in the corresponding static subjective vertical, it seems likely that a reduced otolith gain has larger impact in dynamic situations, provoking postural instability and SIC. According to a significant decrease of the time constant of the velocity storage mechanism, centrifugation affected the otolith-canal interaction at a central level. Admittedly speculative, this fits in the assumption that an earth-anomalous level of gravity has consequences for the determination of the subjective vertical. The gain of the torsional VOR was found to be augmented by 10% after centrifugation, irrespective of body orientation with respect to gravity. Possibly, the observed effects on the VOR may explain the sensations of illusory surround motion during head movements. It is unclear, however, whether these small effects could induce severe symptoms of SIC for several hours afterwards. The vestibular parameters did not have any predictive value for the susceptibility to SIC.
Acknowledgments
This study was financially supported by the Foundation for Behaviourial and Educational Sciences of the Netherlands Organization for Scientific Research (NWO). The costs for the centrifuge run were subsidized by the Stichting Ruimteonderzoek Nederland (SRON)